Curcumin ameliorates oxaliplatin-induced allodynia response and melanocortin downregulation in the spinal cord
DOI:
https://doi.org/10.18413/rrpharmacology.10.478Abstract
Introduction: Oxaliplatin is a platinum-based chemotherapy agent that often causes chemotherapy-induced peripheral neuropathy (CIPN). This effect limits the potential activity and decreases the cancer patient’s quality of life. Melanocortin and transient receptor potential ankyrin 1 (TRPA1) pathways are believed to be essential in recruiting an allodynia response. Based on previous studies, curcumin has shown antioxidant and anti-inflammatory activities that could potentially be useful for decreasing the allodynia effects of oxaliplatin treatment. This study investigated the effect of curcumin on CIPN conditions. In addition, we further elaborated to measure the involvement of spinal melanocortin and the TRPA1 system.
Materials and Methods: A total of 30 male Balb/C mice aged 6-7 weeks old and weighing 20-30 g were used in this study. Mice were injected with oxaliplatin 3 mg/kg four times in the first week of the study. In the second week of the study, curcumin 30, 60, and 120 mg/kg was injected intraperitoneally for 7 days. Allodynia response was measured using the von Frey filament test. Melanocortin 4 receptor (Mc4r), Pro-opiomelanocortin (Pomc) and Trpa1 mRNA expressions were measured using RT-qPCR.
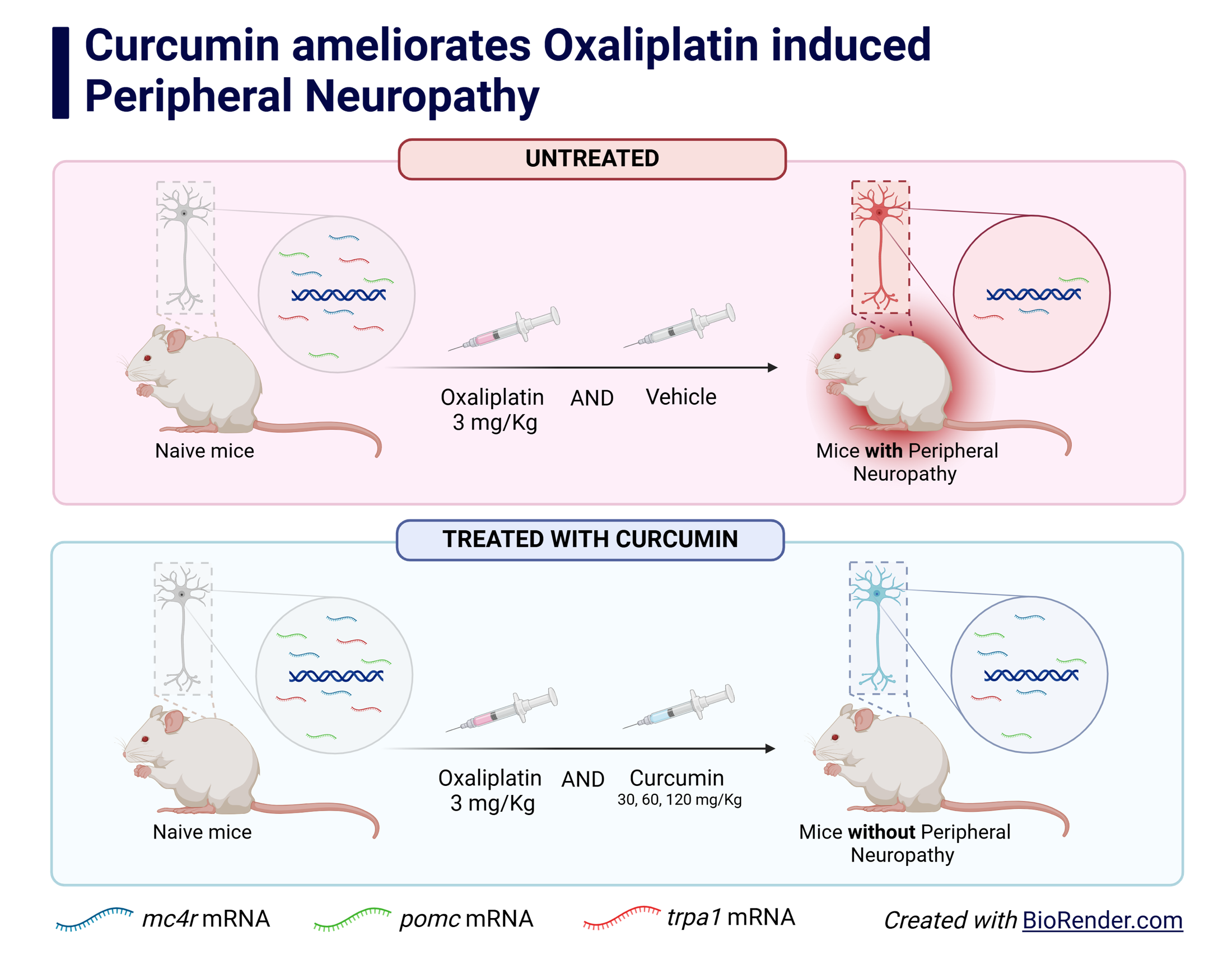
Results: Oxaliplatin-induced mechanical allodynia response in mice, characterized by a decrease in the 50% withdrawal threshold parameter, was followed by a significant decrease in the Mc4r, Pomc, and Trpa1 mRNA expressions in the spinal cord. Curcumin administration in all doses improves the 50% withdrawal threshold parameter in mice induced by oxaliplatin. Furthermore, curcumin increases the Mc4r and Pomc, but not the Trpa1 mRNA expressions in the spinal cord.
Conclusion: Curcumin significantly reduces the allodynia response induced by oxaliplatin. In addition, curcumin ameliorates the melanocortin, but not TRPA1, downregulation in the spinal cord.
Graphical Abstract
Keywords:
Allodynia, Cancer, CIPN, Curcumin, Melanocortin, Neuropathy, Oxaliplatin, TRPA1References
Ardianto C, Judoko AL, Aprilia M, Ratri DMN, Ariyani T, Rahmadi M, Khotib J (2022) Effect of andrographolide and resveratrol on OX1R and prepro-orexin mRNA expression in CIPN-induced hypothalamus of mice with oxaliplatin. Research Journal of Pharmacy and Technology 15(10): 4765–4771. https://doi.org/10.52711/0974-360X.2022.00800
Ardianto C, Lestari D, Primadani LH, Puspitasari DR, Sumartha INB, Nisak K, Budiatin AS, Shinta DW, Andarsari MR, Ifadotunnikmah F, Abdullah ADI, Rahmadi M, Khotib J (2023) Quercetin exerts a protective effect on ischemic stroke-induced memory deficits in mice. Journal of Pharmacology and Pharmacotherapeutics 14(2): 133–141. https://doi.org/10.1177/0976500X231189343
Babu A, Prasanth KG, Balaji B (2015) Effect of curcumin in mice model of vincristine-induced neuropathy. Pharmaceutical Biology 53(6): 838–848. https://doi.org/10.3109/13880209.2014.943247 [PubMed]
Beskhmelnitsyna E, Korokin M, Avtina T, Martynova O, Varavin I, Tishin A (2015) Ion channel TRPA1 is a promising therapeutic target for treatment of pain. Research Results in Pharmacology 1(1): 20–22. https://doi.org/10.18413/2500-235X-2015-1-4-21-24
Caspani O, Zurborg S, Labuz D, Heppenstall PA (2009) The contribution of TRPM8 and TRPA1 channels to cold allodynia and neuropathic pain. PloS One 4(10): e7383. https://doi.org/10.1371/journal.pone.0007383 [PubMed] [PMC]
Chu H, Xia J, Yang Z, Gao J (2012) Melanocortin 4 receptor induces hyperalgesia and allodynia after chronic constriction injury by activation of p38 MAPK in DRG. The International Journal of Neuroscience 122(2): 74–81. https://doi.org/10.3109/00207454.2011.630542 [PubMed]
Di Cesare Mannelli L, Pacini A, Micheli L, Tani A, Zanardelli M, Ghelardini C (2014) Glial role in oxaliplatin-induced neuropathic pain. Experimental Neurology 261: 22–33. https://doi.org/10.1016/j.expneurol.2014.06.016 [PubMed]
Fan X, Zhang C, Liu DB, Yan J, Liang HP (2013) The clinical applications of curcumin: current state and the future. Current Pharmaceutical Design 19(11): 2011–2031. https://doi.org/10.2174/1381612811319110005 [PubMed]
Hopkins HL, Duggett NA, Flatters SJL (2016) Chemotherapy-induced painful neuropathy: pain-like behaviours in rodent models and their response to commonly used analgesics. Current Opinion in Supportive and Palliative Care 10(2): 119–128. https://doi.org/10.1097/SPC.0000000000000204 [PubMed] [PMC]
Jensen TS, Finnerup NB (2014) Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. The Lancet. Neurology 13(9): 924–935. https://doi.org/10.1016/S1474-4422(14)70102-4 [PubMed]
Kandhare AD, Raygude KS, Ghosh P, Ghule AE, Bodhankar SL (2012) Therapeutic role of curcumin in prevention of biochemical and behavioral aberration induced by alcoholic neuropathy in laboratory animals. Neuroscience Letters 511(1): 18–22. https://doi.org/10.1016/j.neulet.2012.01.019 [PubMed]
Katsura H, Obata K, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Sakagami M, Noguchi K (2006) Antisense knock down of TRPA1, but not TRPM8, alleviates cold hyperalgesia after spinal nerve ligation in rats. Experimental Neurology 200(1): 112–123. https://doi.org/10.1016/j.expneurol.2006.01.031 [PubMed]
Kim E (2020) Chemotherapy-induced peripheral neuropathy: bench to clinical practice. The Korean Journal of Pain 33(4): 291–293. https://doi.org/10.3344/kjp.2020.33.4.291[PubMed] [PMC]
Korczeniewska OA, Kohli D, Katzmann Rider G, Zaror C, Iturriaga V, Benoliel R (2021) Effects of melanocortin-4 receptor (MC4R) antagonist on neuropathic pain hypersensitivity in rats - A systematic review and meta-analysis. European Journal of Oral Sciences 129(4): e12786. https://doi.org/10.1111/eos.12786 [PubMed]
Lau JKY, Tian M, Shen Y, Lau SF, Fu WY, Fu AKY, Ip NY (2021) Melanocortin receptor activation alleviates amyloid pathology and glial reactivity in an Alzheimer’s disease transgenic mouse model. Scientific Reports 11(1): 4359. https://doi.org/10.1038/s41598-021-83932-4[PubMed] [PMC]
Li Y, Kim WM, Kim SH, You HE, Kang DH, Lee HG, Choi JI, Yoon MH (2021) Prostaglandin D2 contributes to cisplatin-induced neuropathic pain in rats via DP2 receptor in the spinal cord. The Korean Journal of Pain 34(1): 27–34. https://doi.org/10.3344/kjp.2021.34.1.27[PubMed] [PMC]
Li ZX, Liu BW, He ZG, Xiang HB (2017) Melanocortin-4 receptor regulation of pain. Biochimica et biophysica acta. Molecular Basis of Disease 1863(10 Pt A): 2515–2522. https://doi.org/10.1016/j.bbadis.2017.05.021 [PubMed]
Lin H, Heo BH, Yoon MH (2015) A new rat model of cisplatin-induced neuropathic pain. The Korean Journal of Pain 28(4): 236–243. https://doi.org/10.3344/kjp.2015.28.4.236[PubMed] [PMC]
Marcotti A, Fernández-Trillo J, González A, Vizcaíno-Escoto M, Ros-Arlanzón P, Romero L, Vela JM, Gomis A, Viana F, de la Peña E (2023) TRPA1 modulation by Sigma-1 receptor prevents oxaliplatin-induced painful peripheral neuropathy. Brain 146(2): 475–491. https://doi.org/10.1093/brain/awac273 [PubMed] [PMC]
Mittal N, Joshi R, Hota D, Chakrabarti A (2009) Evaluation of antihyperalgesic effect of curcumin on formalin-induced orofacial pain in rat. Phytotherapy Research 23(4): 507–512. https://doi.org/10.1002/ptr.2662 [PubMed]
Padilla SL, Reef D, Zeltser LM (2012) Defining POMC neurons using transgenic reagents: impact of transient Pomc expression in diverse immature neuronal populations. Endocrinology 153(3): 1219–1231. https://doi.org/10.1210/en.2011-1665 [PubMed] [PMC]
Park JH, Chae J, Roh K, Kil EJ, Lee M, Auh CK, Lee MA, Yeom CH, Lee S (2015) Oxaliplatin-induced peripheral neuropathy via trpa1 stimulation in mice dorsal root ganglion is correlated with aluminum accumulation. PloS One 10(4): e0124875. https://doi.org/10.1371/journal.pone.0124875 [PubMed] [PMC]
Sharfman N, Gilpin NW (2021) The role of melanocortin plasticity in pain-related outcomes after alcohol exposure. Frontiers in Psychiatry 12: 764720. https://doi.org/10.3389/fpsyt.2021.764720 [PubMed] [PMC]
Souza Monteiro de Araujo D, Nassini R, Geppetti P, De Logu F (2020) TRPA1 as a therapeutic target for nociceptive pain. Expert Opinion on Therapeutic Targets 24(10): 997–1008. https://doi.org/10.1080/14728222.2020.1815191 [PubMed] [PMC]
Staff NP, Grisold A, Grisold W, Windebank AJ (2017) Chemotherapy-induced peripheral neuropathy: A current review. Annals of Neurology 81(6): 772–781. https://doi.org/10.1002/ana.24951 [PubMed] [PMC]
Staaf S, Oerther S, Lucas G, Mattsson JP, Ernfors P (2009) Differential regulation of TRP channels in a rat model of neuropathic pain. Pain 144(1-2): 187–199. https://doi.org/10.1016/j.pain.2009.04.013 [PubMed]
Starowicz K, Bilecki W, Sieja A, Przewlocka B, Przewlocki R (2004) Melanocortin 4 receptor is expressed in the dorsal root ganglions and down-regulated in neuropathic rats. Neuroscience Letters 358(2): 79–82. https://doi.org/10.1016/j.neulet.2003.12.096 [PubMed]
Waseem M, Parvez S (2016) Neuroprotective activities of curcumin and quercetin with potential relevance to mitochondrial dysfunction induced by oxaliplatin. Protoplasma 253(2): 417–430. https://doi.org/10.1007/s00709-015-0821-6 [PubMed]
Yardım A, Kandemir FM, Çomaklı S, Özdemir S, Caglayan C, Kucukler S, Çelik H (2021) Protective effects of curcumin against paclitaxel-induced spinal cord and sciatic nerve injuries in rats. Neurochemical Research 46(2): 379–395. https://doi.org/10.1007/s11064-020-03174-0 [PubMed]
Zajączkowska R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J (2019) Mechanisms of chemotherapy-induced peripheral neuropathy. International Journal of Molecular Sciences 20(6): 1451. https://doi.org/10.3390/ijms20061451 [PubMed] [PMC]
Zhao Y, Xin Y, Chu H (2019) MC4R is involved in neuropathic pain by regulating JNK signaling pathway after chronic constriction injury. Frontiers in Neuroscience 13: 919. https://doi.org/10.3389/fnins.2019.00919 [PubMed] [PMC]
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Alma Nuril Aliya, Luke Wongso, Diah Ayu Wakita Trimanda, Risda Maulida, I Nengah Budi Sumartha, Samirah Samirah, Abdulloh Machin, Long Chiau Ming, Abdelhakim Bouyahya, Mahardian Rahmadi, Junaidi Khotib, Сhrismawan Ardianto

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

