Cytotoxic and cytostatic activity of five new imidazotetrazine derivatives on breast cancer cell cultures MDAMB231, BT474, and MCF-7
DOI:
https://doi.org/10.18413/rrpharmacology.10.479Abstract
Introduction: The work presents the results of the study of new imidazotetrazine derivatives to establish the possibility of using them as anticancer agents, including for chemotherapy of metastatic breast cancer. The relevance of the work is due to the wide spread of oncological diseases and high cancer mortality, which dictates the need to constantly obtain cell lines and improve cultivation protocols for testing new antitumor drugs. The goal of this study is to check the potential of five new imidazotetrazine derivatives to become new antitumor drugs, in the scope of studying their cytotoxic and cytostatic activities on breast cancer cell cultures.
Materials and Methods: The culturing MCF-7, MDAMB231, BT474, and MCF-10a cells with determining cytotoxic and cytostatic activities of five new azolotetrazine derivatives are base methods used in this study.
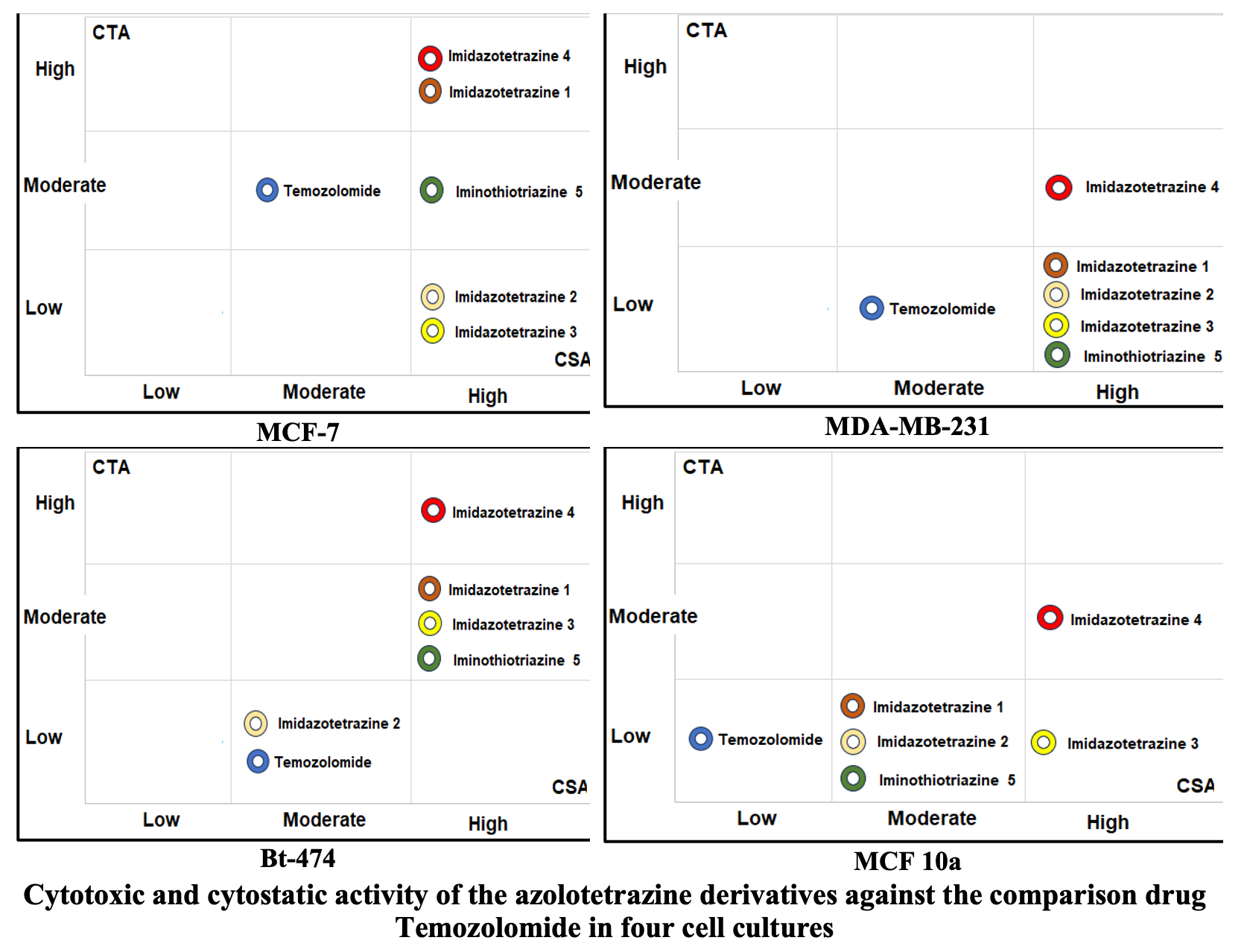
Results: For the MCF-7 culture, MCS of comparison drug temozolomide was equal to 2.44 and IC50 was 6.81 mM/L; for other cultures CTA indicators were worse. Imidazotetrazine 2 and imidazotetrazine 3 demonstrated CTA indicators lower than those of temozolomide. IC50 was not achieved, and the MCS value varied between 1.34 and 1.74. These two derivatives were classified as the compounds with an extremely low CTA. Imidazotetrazine 1 and iminothiotriazine 5 showed cytotoxic activity higher than that of the comparison drug and we classified these compounds as the ones with a moderate CTA. Finally, we found imidazotetrazine 4 with IC50 of 0.85 mM/L and CTA of 7.34 as a compound with a potentially strong anticancer effect for further investigation. The cytostatic activity of four of the five azoloazine derivatives studied was in a narrow range corresponding to the survival rate from 0.21 to 0.32, depending on the compound and cell culture. Against this background, imidazotetrazine 4 demonstrated a higher CSA, determined by the survival rate from 0.17 to 0.20.
Conclusion: As a result of an in vitro study, we found that five new azolotriazine derivatives can be evaluated in the ascending order of these properties, as a combination of CTA+CSA in order imidazotetrazine 2, imidazotetrazine 3 < temozolomide < imidazotetrazine 1, iminothiotriazine 5 < imidazotetrazine 4, although the CSA of all the studied compounds turned out to be high. Thus, 3-Cyclohexyl-4-oxoimidazo[5,1-d]-[1,2,3,5]tetrazine-8-N-piperidinyl-carboxamide (imidazotetrazine 4) is an unconditional leader in the tested series of new azoloazine derivatives and we recommend it for further preclinical trials.
Graphical Abstract
Keywords:
breast cancer, imidazotetrazine, cytotoxic activity, cytostatic activity, MCF-7 cell line, MDAMB231 cell line, BT474 cell line, MCF-10a cell lineReferences
Akram M, Iqbal M, Daniyal M, Khan AU (2017) Awareness and current knowledge of breast cancer. Biological Research 50(1): 33. https://doi.org/10.1186/s40659-017-0140-9 [PubMed] [PMC]
Alexandrova R, Dinev D, Gavrilova-Valchеva I, Gavrilov I (2019) Cell cultures as model systems in breast cancer research. Merit Research Journal of Medicine and Medical Sciences 7(2): 73–79.
Arnedos M, Vicier C, Loi S, Lefebvre C, Michiels S, Bonnefoi H, Andre F (2015) Precision medicine for metastatic breast cancer-limitations and solutions. Nature Reviews Clinical Oncology 12(12): 693–704. https://doi.org/10.1038/nrclinonc.2015.123 [PubMed]
Azamjah N, Soltan-Zadeh Y, Zayeri F (2019) Global trend of breast cancer mortality rate: a 25-year study. The Asian Pacific Journal of Cancer Prevention 20(7): 2015–2020. https://doi.org/10.31557/APJCP.2019.20.7.2015 [PubMed] [PMC]
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians 68(6): 394–424. https://doi.org/10.3322/caac.21492 [PubMed]
Clegg J, Koch MK, Thompson EW, Haupt LM, Kalita-de Croft P, Bray LJ (2020) Three-dimensional models as a new frontier for studying the role of proteoglycans in the normal and malignant breast microenvironment. Frontiers in Cell and Developmental Biology 8: 569454. https://doi.org/10.3389/fcell.2020.569454 [PubMed] [PMC]
Comşa Ş, Cîmpean AM, Raica M (2015) The story of MCF-7 breast cancer cell line: 40 years of experience in research. Anticancer Research 35(36): 3147–3154. [PubMed]
Dai X, Cheng H, Bai Z, Li J (2017) Breast cancer cell line classification and its relevance with breast tumor subtyping. Journal of Cancer 8(16): 3131–3141. https://doi.org/10.7150/jca.18457 [PubMed] [PMC]
Dwyer MP, Keertikar K, Paruch K, Alvarez C, Labroli M, Poker C, Fischmann TO, Mayer-Ezell R, Bond R, Wang Y, Azevedo R, Guzi TJ (2013) Discovery of pyrazolo[1,5-a]pyrimidine-based Pim inhibitors: a template-based approach. Bioorganic & Medicinal Chemistry Letters 23(22): 6178–6182. https://doi.org/10.1016/j.bmcl.2013.08.110 [PubMed]
Feiten S, Dünnebacke J, Heymanns J, Köppler H, Thomalla J, Roye C, Wey D, Weide R (2014) Breast cancer morbidity. Deutsches Ärzteblatt International 111: 537–544. https://doi.org/10.3238/arztebl.2014.0537 [PubMed] [PMC]
Garza-Morales R, Gonzalez-Ramos R, Chiba A, Montes de Oca-Luna R, McNally LR, McMasters KM, Gomez-Gutierrez JG (2018) Temozolomide enhances triple-negative breast cancer virotherapy in vitro. Cancers (Basel) 10(5): 144. https://doi.org/10.3390/cancers10050144 [PubMed] [PMC]
Hassan MS, Ansari J, Spooner D, Hussain SA (2010) Chemotherapy for breast cancer (Review). Oncology Reports 24(5): 1121–1131. https://doi.org/10.3892/or_00000963
Holliday DL, Speirs V (2011) Choosing the right cell line for breast cancer research. Breast Cancer Research 13(4): 215. https://doi.org/10.1186/bcr2889 [PubMed] [PMC]
Horishny V, Mandzyuk L, Lytvyn R, Bodnarchuk OV, Matiychuk VS, Obushak MD (2020) Synthesis and biological activity of pyrazolo[1,5-c][1,3]benzoxazines containing a tiazolidin-4-one fragment. Russian Journal of Organic Chemistry 56(4): 588–595. https://doi.org/10.1134/S1070428020040053
Khodadadi A, Faghih-Mirzaei E, Karimi-Maleh H, Abbaspourrad A, Agarwal S, Gupta VK (2019) A new epirubicin biosensor based on amplifying DNA interactions with polypyrrole and nitrogen-doped reduced graphene: experimental and docking theoretical investigations. Sensors and Actuators B: Chemical 284: 568–574. https://doi.org/10.1016/j.snb.2018.12.164
Mansinho A, Boni V, Miguel M, Calvo E (2019) New designs in early clinical drug development. Annals of Oncology 30(9): 1460–1465. https://doi.org/10.1093/annonc/mdz191 [PubMed]
Moody C, Wheelhouse R (2014) The medicinal chemistry of imidazotetrazine prodrugs. Pharmaceuticals (Basel) 7(7): 797–838. https://doi.org/10.3390/ph7070797 [PubMed] [PMC]
Nakhjavani M, Shirazi FH (2017) Reporting the effect of cell seeding density on growth pattern of cancer cell lines. Iranian Journal of Pharmaceutical Sciences 13(2): 87–94. https://doi.org/10.22037/ijps.v13.40700
Pakina VA, Iksanova EZ, Shih EV, Tumutolova OМ, Arutiunian KK, Kargina IV, Blinov KD, Pilgaev FP, Epishkina AA, Blinov DS, Grebenkin EV, Blinova EV (2024) An effective way for targeting EGFR-mediated carcinogenesis: an in vitro study. Research Results in Pharmacology 10(2): 17–26. https://doi.org/10.18413/rrpharmacology.10.453
Rositch AF, Unger-Saldaña K, DeBoer RJ, Ng’ang’a A, Weiner BJ (2020) The role of dissemination and implementation science in global breast cancer control programs: Frameworks, methods, and examples. Cancer 126 (10): 2394–2404. https://doi.org/10.1002/cncr.32877 [PubMed]
Sadchikova E, Bakulev V, Subbotina J, Privalova D, Dehaen W, Hecke K, Robeyns K, Meervelt LV, Mokrushin VS(2013) ChemInform abstract: synthesis and structure of new imidazo- and pyrazolo[5,1-d][1,2,3,5]thiatriazines based on the reaction of diazoazoles with acyl isothiocyanates controlled by SO interaction. Tetrahedron 69: 6987–6992. https://doi.org/10.1016/j.tet.2013.06.062
Sadchikova EV (2016) Synthesis of new azolo[5,1-d][1,2,3,5]tetrazin-4-ones – analogs of antitumor agent temozolomide. Russian Chemical Bulletin 65: 1867–1872. https://doi.org/10.1007/s11172-016-1522-9
Shirazi F, Zarghi A, Kobarfard F, Zendehdel R, Nakhjavani M, Arfaiee S, et al. (2011) Remarks in successful cellular investigations for fighting breast cancer using novel synthetic compounds. In: Gunduz M, Gunduz E (Eds) Breast Cancer-focusing Tumor Microenvironment, Stem Cells and Metastasis. InTech, 85–102. https://doi.org/10.5772/23005
Stockert JC, Horobin RW, Colombo LL, Blázquez-Castro A (2018) Tetrazolium salts and formazan products in cell biology: viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochemica 120(3): 159–167. https://doi.org/10.1016/j.acthis.2018.02.005 [PubMed]
Sztanke K, Pasternak K, Rzymowska J, Sztanke M, Kandefer-Szerszeń M (2008) Synthesis, structure elucidation and identification of antitumoural properties of novel fused 1,2,4-triazine aryl derivatives. European Journal of Medicinal Chemistry 43(5): 1085–1094. https://doi.org/10.1016/j.ejmech.2007.07.009 [PubMed]
Vajrabhaya L, Korsuwannawong S (2018) Cytotoxicity evaluation of a Thai herb using tetrazolium (MTT) and sulforhodamine B (SRB) assays. Journal of Analytical Science and Technology 9: 15 https://doi.org/10.1186/s40543-018-0146-0
Wang X, Luo N, Xu Zh, Zheng X, Huang B, Pan X (2020) The estrogenic proliferative effects of two alkylphenols and a preliminary mechanism exploration in MCF-7 breast cancer cells. Environmental Toxicology 35(5): 628–638. https://doi.org/10.1002/tox.22898 [PubMed]
Wilkinson L, Gathani T (2022) Understanding breast cancer as a global health concern. The British Journal of Radiology 95(1130): 20211033. https://doi.org/10.1259/bjr.20211033 [PubMed] [PMC]
Yan S, Yue S (2023) Identification of early diagnostic biomarkers for breast cancer through bioinformatics analysis. Medicine (Baltimore). 102(37): e35273. https://doi.org/10.1097/MD.0000000000035273 [PubMed] [PMC]
Zhang KP, Fang X, Zhang Y, Chao M (2021) The prognosis of cancer patients undergoing liposomal doxorubicin-based chemotherapy: A systematic review and meta-analysis. Medicine (Baltimore) 100(34): https://doi.org/10.1097/MD.0000000000026690 [PubMed] [PMC]
Zhukova LG, Andreeva II, Zavalishina LE, Zakiriakhodzhaev AD, Koroleva IA, Nazarenko AV, Paltuev RM, Parokonnaia AA, Petrovskii AV, Portnoi SM, Semiglazov VF, Semiglazova TI, Stenina MB, Stepanova AM, Trofimova OP, Tyulyandin SA, Frank GA, Frolova MA, Shatova IS, Nevol’skikh AA, Ivanov SA, Khailova ZV, Gevorkian TG (2021) Breast cancer. Journal of Modern Oncology [Sovremennaya Onkologiya] 23(1): 5–40. https://doi.org/10.26442/18151434.2021.1.200823
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Ahmed Hamid Al-Humairi, Svetlana E. Sitnikova, Valery V. Novochadov

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

