CAR-T cells: prospective genetic engineering approach to orchestrate solid tumor in lung cancer
DOI:
https://doi.org/10.18413/rrpharmacology.10.522Abstract
Introduction: Various strategies, starting with surgery, systemic radiation therapy with cytotoxic chemotherapy, and immunotherapy, have been carried out to address mortality from lung cancer. None of these therapies has showed remarkably successful treatments for lung cancer. Developing technology leads to innovation of cancer-targeted therapy as an ideal strategy. Chimeric antigen receptor T cells (CAR-T cells) are one of the novel immunotherapy approaches in genetic engineering. CAR-T therapy has showed promising results as the cancer-targeted therapy.
Methods: This review identifies relevant research by the keywords “Lung Cancer”, “CAR-T for Lung Cancer”, “CAR-T for solid tumor”, “CAR-T for SCLC” or “CAR-T for NSCLC”. The articles are extracted from Pubmed, Web of Science, Scopus, Science Direct, and Google Scholar. Data are shown based on discussions of the modification receptor, co-stimulator, and CAR-T cells.
Results: CAR-T acts to overcome lung cancer by targeting several receptors, such as mesothelin and c-Met. Several generations have also evolved to more specific and effective therapy. CAR-T modifications like BBIR, SUPRA CAR, and UIR have also been developed to minimize side effects.
Conclusion: CAR-T developments are not limited to CAR modification, but include its components and structure.
Graphical Abstract
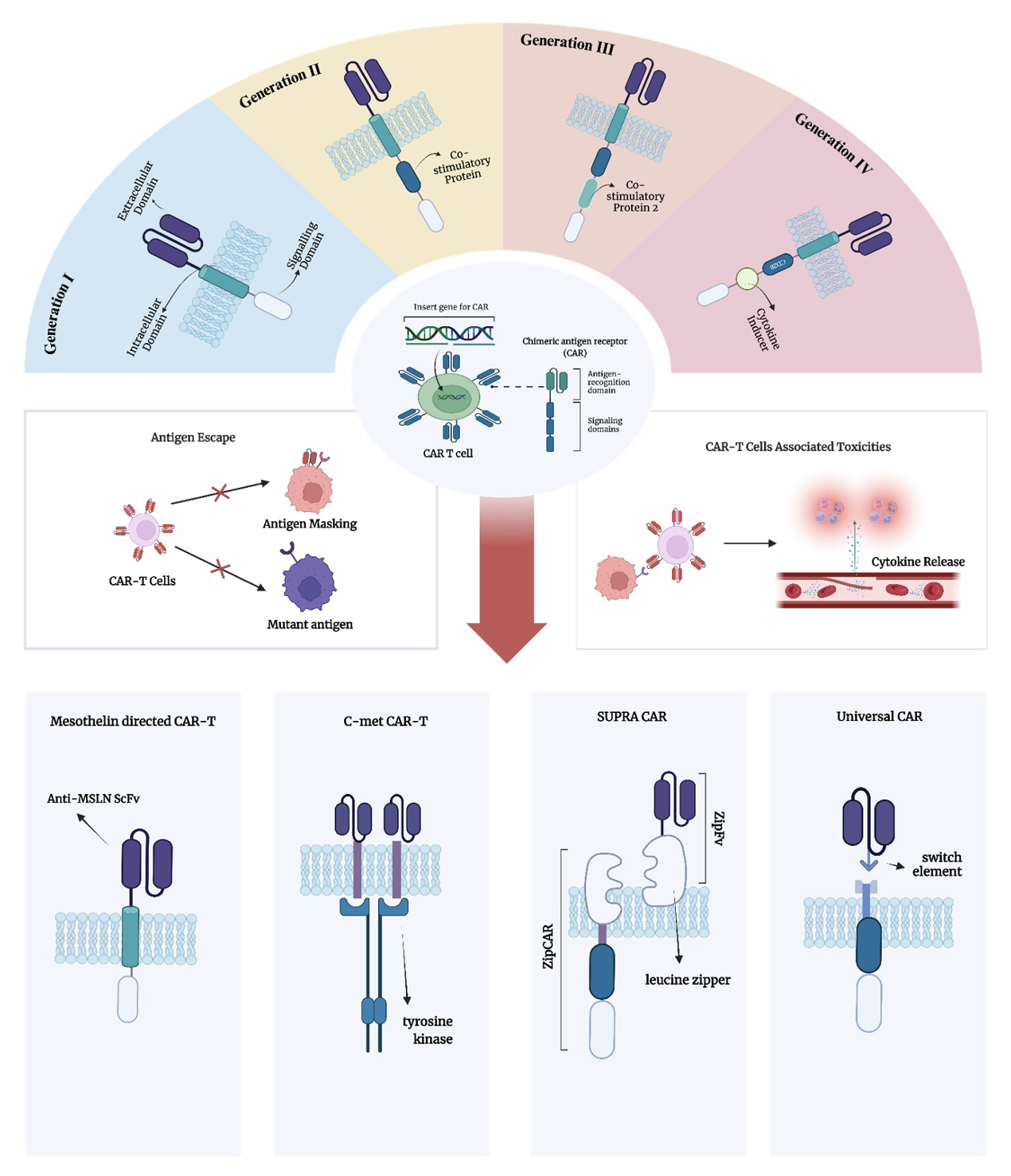
Keywords:
Cancer, Lung Cancer, Solid Tumor, Chimeric Antigen Receptor, CAR-T Modification, Genetic EngineeringReferences
Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, Mehta A, Purev E, Maloney DG, Andreadis C, Sehgal A, Solomon SR, Ghosh N, Albertson TM, Garcia J, Kostic A, Mallaney M, Ogasawara K, Newhall K, Kim Y, Li D, Siddiqi T (2020) Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. The Lancet 396(10254): 839–852. https://doi.org/10.1016/S0140-6736(20)31366-0 [PubMed]
Adusumilli PS, Cherkassky L, Villena-Vargas J, Colovos C, Servais E, Plotkin J, Jones DR, Sadelain M (2014) Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Science Translational Medicine 6(261): 261ra151–261ra151. https://doi.org/10.1126/scitranslmed.3010162 [PubMed] [PMC]
Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP; CONCORD Working Group (2018) Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. The Lancet 391(10125): 1023–1075. https://doi.org/10.1016/S0140-6736(17)33326-3 [PubMed] [PMC]
Awad MM, Oxnard GR, Jackman DM, Savukoski DO, Hall D, Shivdasani P, Heng JC, Dahlberg SE, Jänne PA, Verma S, Christensen J, Hammerman PS, Sholl LM (2016) MET exon 14 mutations in non–small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. Journal of Clinical Oncology 34(7): 721–730. https://doi.org/10.1200/JCO.2015.63.460 [PubMed]
Baker S, Dahele M, Lagerwaard FJ, Senan S (2016) A critical review of recent developments in radiotherapy for non-small cell lung cancer. Radiation Oncology 11(1): 1–14. https://doi.org/10.1186/s13014-016-0693-8 [PubMed] [PMC]
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A (2024) Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 74(3): 229–263. https://doi.org/10.3322/caac.21834 [PubMed]
Brocker T (2000) Chimeric Fv-zeta or Fv-epsilon receptors are not sufficient to induce activation or cytokine production in peripheral T cells. Blood, The Journal of the American Society of Hematology 96(5): 1999–2001. https://doi.org/10.1182/blood.V96.5.1999[PubMed]
Car BD, Eng VM, Lipman JM, Anderson TD (1999) The toxicology of interleukin-12: a review. Toxicologic Pathology 27(1): 58–63. https://doi.org/10.1177/019262339902700112 [PubMed]
Castelletti L, Yeo D, van Zandwijk N, Rasko JE (2021) Anti-Mesothelin CAR T cell therapy for malignant mesothelioma. Biomarker Research 9(1): 1–13. https://doi.org/10.1186/s40364-021-00264-1 [PubMed] [PMC]
Chmielewski M, Abken H (2020) TRUCKS, the fourth‐generation CAR T cells: current developments and clinical translation. Advances in Cell and Gene Therapy 3(3): e84. https://doi.org/10.1002/acg2.84
Cho JH, Collins JJ, Wong WW (2018) Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell 173(6): 1426–1438. https://doi.org/10.1016/j.cell.2018.03.038 [PubMed] [PMC]
Clémenceau B, Valsesia-Wittmann S, Jallas AC, Vivien R, Rousseau R, Marabelle A, Caux C, Vié H (2015) In vitro and in vivo comparison of lymphocytes transduced with a human CD16 or with a chimeric antigen receptor reveals potential off‐target interactions due to the IgG2 CH2‐CH3 CAR‐ Journal of Immunology Research 2015(1): 482089. https://doi.org/10.1155/2015/482089 [PubMed] [PMC]
Debela DT, Muzazu SG, Heraro KD, Ndalama MT, Mesele BW, Haile DC, Kitui SK, Manyazewal T (2021) New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Medicine 9: 20503121211034366. https://doi.org/10.1177/20503121211034366 [PubMed] [PMC]
Fajgenbaum DC, June CH (2020) Cytokine storm. The New England Journal of Medicine 383(23): 2255–2273. https://doi.org/10.1056/NEJMra2026131 [PubMed]Godoy LA, Chen J, Ma W, Lally J, Toomey KA, Rajappa P, Sheridan R, Mahajan S, Stollenwerk N, Phan CT, Cheng D, Knebel RJ, Li T (2023) Emerging precision neoadjuvant systemic therapy for patients with resectable non-small cell lung cancer: current status and perspectives. Biomarker Research 11(1): 7. https://doi.org/10.1186/s40364-022-00444-7 [PubMed] [PMC]
Gorovits B, Koren E (2019) Immunogenicity of chimeric antigen receptor T-cell therapeutics. BioDrugs 33(3): 275–284. https://doi.org/10.1007/s40259-019-00354-5[PubMed]
Grossman R, Haim O, Abramov S, Shofty B, Artzi M (2021) Differentiating small-cell lung cancer from non-small-cell lung cancer brain metastases based on MRI using efficientnet and transfer learning approach. Technology in Cancer Research & Treatment 20: 15330338211004919. https://doi.org/10.1177/15330338211004919 [PubMed] [PMC]
Gun SY, Lee SWL, Sieow JL, Wong SC (2019) Targeting immune cells for cancer therapy. Redox Biology 25: 101174. https://doi.org/10.1016/j.redox.2019.101174 [PubMed] [PMC]
Hodge G, Barnawi J, Jurisevic C, Moffat D, Holmes M, Reynolds PN, Reynolds PN, Hodge S (2014) Lung cancer is associated with decreased expression of perforin, granzyme B and interferon (IFN)-γ by infiltrating lung tissue T cells, natural killer (NK) T-like and NK cells. Clinical and Experimental Immunology 178(1): 79–85. https://doi.org/10.1111/cei.12392[PubMed] [PMC]
Holstein SA, Lunning MA (2020) CAR T‐cell therapy in hematologic malignancies: a voyage in progress. Clinical Pharmacology & Therapeutics 107(1): 112–122. https://doi.org/10.1002/cpt.1674 [PubMed]
Kachala SS, Bograd AJ, Villena-Vargas J, Suzuki K, Servais EL, Kadota K, Chou J, Sima CS, Vertes E, Rusch VW, Travis WD, Sadelain M, Adusumili PS (2014) Mesothelin overexpression is a marker of tumor aggressiveness and is associated with reduced recurrence-free and overall survival in early-stage lung adenocarcinoma. Clinical Cancer Research 20(4): 1020–1028. https://10.1158/1078-0432.CCR-13-1862 [PubMed] [PMC]
Kandra P, Nandigama R, Eul B, Huber M, Kobold S, Seeger W, Grimminger F, Savai R (2022) Utility and drawbacks of chimeric antigen receptor T cell (CAR-T) therapy in lung cancer. Frontiers in Immunology 13: 903562. https://doi.org/10.3389/fimmu.2022.903562[PubMed] [PMC]
Kim KH, Kim H (2017) Progress of antibody-based inhibitors of the HGF–cMET axis in cancer therapy. Experimental and Molecular Medicine 49(3): e307–e307. https://doi.org/10.1038/emm.2017.17 [PubMed] [PMC]
Lee III DW, Stetler-Stevenson M, Yuan CM, Fry TJ, Shah NN, Delbrook C, Yates B, Zhang H, Zhang L, Kochenderfer JN, Rosenberg SA, Stroncek D, Mackall CL (2015) Safety and response of incorporating CD19 chimeric antigen receptor T cell therapy in typical salvage regimens for children and young adults with acute lymphoblastic leukemia. Blood 126(23): 684. https://doi.org/10.1182/blood.V126.23.684.684
Li C, Lei S, Ding L, Xu Y, Wu X, Wang H, Zhang Z, Gao T, Zhang Y, Li L (2023) Global burden and trends of lung cancer incidence and mortality. Chinese Medical Journal 136(13): 1583–1590. https://doi.org/10.1097/CM9.0000000000002529 [PubMed] [PMC]
Liang H, Wang M (2020) MET oncogene in non-small cell lung cancer: mechanism of MET dysregulation and agents targeting the HGF/c-Met axis. OncoTargets Therapy 2491-2510. https://doi.org/10.2147/OTT.S231257 [PubMed] [PMC]
Lipowska-Bhalla G, Gilham DE, Hawkins RE, Rothwell DG (2012) Targeted immunotherapy of cancer with CAR T cells: achievements and challenges. Cancer Immunology, Immunotherapy 61(7): 953–962. https://doi.org/10.1007/s00262-012-1254-0 [PubMed] [PMC]
Liu B, Wu X, Liu B, Wang C, Liu Y, Zhou Q, Xu K (2012) MiR-26a enhances metastasis potential of lung cancer cells via AKT pathway by targeting PTEN. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1822(11): 1692–1704. https://doi.org/10.1016/j.bbadis.2012.07.019 [PubMed]
Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, Lin Y, Braunschweig I, Hill BT, Timmerman JM, Deol A, Reagan PM, Stiff P, Flinn IW, Farooq U, Goy A, McSweeney PA, Munoz J, Siddiqi T, Chavez JC, Herrera AF, Bartlett NL, Wiezorek JS, Navale L, Xue A, Jiang Y, Bot A, Rossi JM, Kim JJ, Go WY, Neelapu SS (2019) Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. The Lancet Oncology 20(1): 31–42. https://doi.org/10.1016/S1470-2045(18)30864-7 [PubMed] [PMC]
Lownik JC, Conrad DH, Martin RK (2020) T cell receptor signaling defines the fate and pathway of ICOS internalization. Biochemistry and Biophysics Reports. 24: 100803. https://doi.org/10.1016/j.bbrep.2020.100803 [PubMed] [PMC]
Marofi F, Motavalli R, Safonov VA, Thangavelu L, Yumashev AV, Alexander M, Shomali N, Chartrand MS, Pathak Y, Jarahian M, Izadi S, Hassanzadeh A, Shirafkan N, Tahmasebi S, Khiavi FM (2021) CAR T cells in solid tumors: challenges and opportunities. Stem Cell Research & Therapy 12(1): 1–16. https://doi.org/10.1186/s13287-020-02128-1 [PubMed] [PMC]
Maude SL, Laetsch TW, Buechner J, Rives S, Boyer,M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, Zhang Y, Sen K, Lebwohl D, Pulsipher MA, Grupp SA (2018) Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. New England Journal of Medicine 378(5): 439–448. https://doi.org/10.1056/NEJMoa1709866 [PubMed] [PMC]
Min J, Long C, Zhang L, Duan J, Fan H, Chu F, Li Z (2022) c-Met specific CAR-T cells as a targeted therapy for non-small cell lung cancer cell A549. Bioengineered 13(4): 9232–9248. https://doi.org/10.1080/21655979.2022.2058149 [PubMed] [PMC]
Minutolo NG, Hollander EE, Powell Jr DJ (2019) The emergence of universal immune receptor T cell therapy for cancer. Frontiers in Oncology 9: 176. https://doi.org/10.3389/fonc.2019.00176 [PubMed] [PMC]
Morello A, Sadelain M, Adusumilli PS (2016) Mesothelin-targeted CARs: driving T cells to solid tumors. Cancer Discovery 6(2): 133–146. https://doi.org/10.1158/2159-8290.CD-15-0583 [PubMed] [PMC]
Moreno C, Haynie C, Johnson A, Weber KS (2022) Alternative CAR therapies: Recent approaches in engineering chimeric antigen receptor immune cells to combat cancer. Biomedicines 10(7): 1493. https://doi.org/10.3390/biomedicines10071493 [PubMed] [PMC]
Neelapu SS, Tummala S, Kebriaei P, Wierda W, Gutierrez C, Locke FL, Komanduri KV, Lin Y, Jain N, Daver N, Westin J, Gulbis AM, Loghin ME, de Groot JF, Adkins S, Davis SE, Rezvani K, Hwu P, Shpall EJ (2018) Chimeric antigen receptor T-cell therapy – assessment and management of toxicities. Nature reviews Clinical Oncology 15(1): 47–62. https://doi.org/10.1038/nrclinonc.2017.148 [PubMed] [PMC]
Park JH, Riviere I, Wang X, Bernal Y, Purdon T, Halton E, Curran JC, Sauter CS, Sadelain M, Brentjens RJ (2015) Efficacy and safety of CD19-targeted 19-28z CAR modified T cells in adult patients with relapsed or refractory B-ALL. Journal of Clinical Oncolology 33(15). https://10.1200/jco.2015.33.15_suppl.701
Popper HH (2016) Progression and metastasis of lung cancer. Cancer and Metastasis Reviews 35(1): 75–91. https://doi.org/10.1007/s10555-016-9618-0 [PubMed] [PMC]
Ramachandran M, Dimberg A, Essand M (2017) The cancer-immunity cycle as rational design for synthetic cancer drugs: Novel DC vaccines and CAR T-cells. In Seminars in Cancer Biology 45: 23–35. https://doi.org/10.1016/j.semcancer.2017.02.010 [PubMed]
Roskoski JrR (2014) The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacological Research 79: 34–74. https://doi.org/10.1016/j.phrs.2013.11.002[PubMed]
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH; Eastern Cooperative Oncology Group (2002) Comparison of four chemotherapy regimens for advanced non–small-cell lung cancer. New England Journal of Medicine 346(2): 92–98. https://doi.org/10.1056/NEJMoa011954 [PubMed]
Soerjomataram I, Cabasag C, Bardot A, Fidler-Benaoudia MM, Miranda-Filho A, Ferlay J, Parkin DM, Ranganathan R, Piñeros M, Znaor A, Mery L, Joko-Fru YW, Dikshit R, Sankaranarayanan R, Swaminathan R, Bray F; SURVCAN-3 collaborators (2023) Cancer survival in Africa, central and south America, and Asia (SURVCAN-3): a population-based benchmarking study in 32 countries. The Lancet Oncology 24(1): 22–32. https://doi.org/10.1016/S1470-2045(22)00704-5 [PubMed]
Sotoudeh M, Shirvani SI, Merat S, Ahmadbeigi N, Naderi M (2019) MSLN (Mesothelin), ANTXR1 (TEM8), and MUC3A are the potent antigenic targets for CAR T cell therapy of gastric adenocarcinoma. Journal of Cellular Biochemistry 120(4): 5010–5017. https://doi.org/10.1002/jcb.27776 [PubMed]
Sterner RC, Sterner RM (2021) CAR-T cell therapy: current limitations and potential strategies. Blood Cancer Journal 11(4): 69. https://doi.org/10.1038/s41408-021-00459-7[PubMed] [PMC]
Styczyński J (2020) A brief history of CAR-T cells: from laboratory to the bedside. Acta Haematologica Polonica 51(1): 2–5. https://doi.org/10.2478/ahp-2020-0002
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 71(3): 209–249. https://doi.org/10.3322/caac.21660 [PubMed]
Tokarew N, Ogonek J, Endres S, von Bergwelt-Baildon M, Kobold S (2019) Teaching an old dog new tricks: next-generation CAR T cells. British Journal of Cancer 120(1): 26–37. https://doi.org/10.1038/s41416-018-0325-1 [PubMed] [PMC]
Tomasik J, Jasiński M, Basak GW (2022) Next generations of CAR-T cells-new therapeutic opportunities in hematology? Frontiers in Immunology 13: 1034707. https://doi.org/10.3389/fimmu.2022.1034707 [PubMed] [PMC]
Urbanska K, Lanitis E, Poussin M, Lynn RC, Gavin BP, Kelderman S, Yu J, Scholler N, Powell Jr DJ (2012) A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Research 72(7): 1844–1852. https://doi.org/10.1158/0008-5472.CAN-11-3890 [PubMed]
Wang S, Tang J, Sun T, Zheng X, Li J, Sun H, Zhou X, Zhou C, Zhang H, Cheng Z, Ma H, Sun H (2017) Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Scientific Reports 7(1): 1339.https://doi.org/10.1038/s41598-017-01571-0 [PubMed] [PMC]
Wolf A, Oeffinger KC, Shih TYC, Walter LC, Church TR, Fontham ET, Elkin EB, Etzioni RD, Guerra CE, Perkins RB, Kondo KK, Kratzer TB, Manassaram-Baptiste D, Dahut WL, Smith RA (2024) Screening for lung cancer: 2023 guideline update from the American Cancer Society. CA: A Cancer Journal for Clinicians 74(1): 50–81. https://doi.org/10.3322/caac.21811 [PubMed]
Wu L, Brzostek J, Sankaran S, Wei Q, Yap J, Tan TYY, Lai J, MacAry PA, Gascoigne NR (2021) Targeting CAR to the peptide-MHC complex reveals distinct signaling compared to that of TCR in a jurkat T cell model. Cancers 13(4): 867. https://doi.org/doi:10.3390/cancers13040867 [PubMed] [PMC]
Xiao X, Huang S, Chen S, Wang Y, Sun Q, Xu X, Li Y (2021) Mechanisms of cytokine release syndrome and neurotoxicity of CAR T-cell therapy and associated prevention and management strategies. Journal of Experimental & Clinical Cancer Research 40(1): 1–23.https://doi.org/10.1186/s13046-021-02148-6 [PubMed] [PMC]
Ye L, Lou Y, Lu L, Fan X (2019) Mesothelin-targeted second generation CAR‑T cells inhibit growth of mesothelin‑expressing tumors in vivo. Experimental and Therapeutic Medicine 17(1): 739–747. https://doi.org/10.3892/etm.2018.7015 [PubMed] [PMC]
Yu X, Ji X, Su C (2022) HER2-altered non-small cell lung cancer: biology, clinicopathologic features, and emerging therapies. Frontiers in Oncology 12: 860313. https://doi.org/10.3389/fonc.2022.860313 [PubMed] [PMC]
Zhang Y, Zhang Z (2020) The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cellular & Molecular Immunology 17(8): 807–821. https://doi.org/10.1038/s41423-020-0488-6 [PubMed] [PMC]
Zhao J, Lin Q, Song Y, Liu D (2018) Universal CARs, universal T cells, and universal CAR T cells. Journal of Hematology & Oncology 11(1): 1–9. https://doi.org/10.1186/s13045-018-0677-2 [PubMed] [PMC]
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Tanujaya AF, Anggraini D, Nurhan AD

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

