The study of systemic exposition of 4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonamide in blood on rats
DOI:
https://doi.org/10.18413/rrpharmacology.11.529Abstract
Introduction: The 4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonamide (ODASA) is new antiglaucoma drug. The compounds similar in structure are able to accumulate in red blood cells. Therefore, it is necessary to evaluate the pharmacokinetic parameters of ODASA and its metabolite in whole blood of laboratory animals. Aim: Calculation of pharmacokineticparameters of ODASA and its metabolites in rat whole blood after ocular instillation andintraperitoneal injection of the drug.
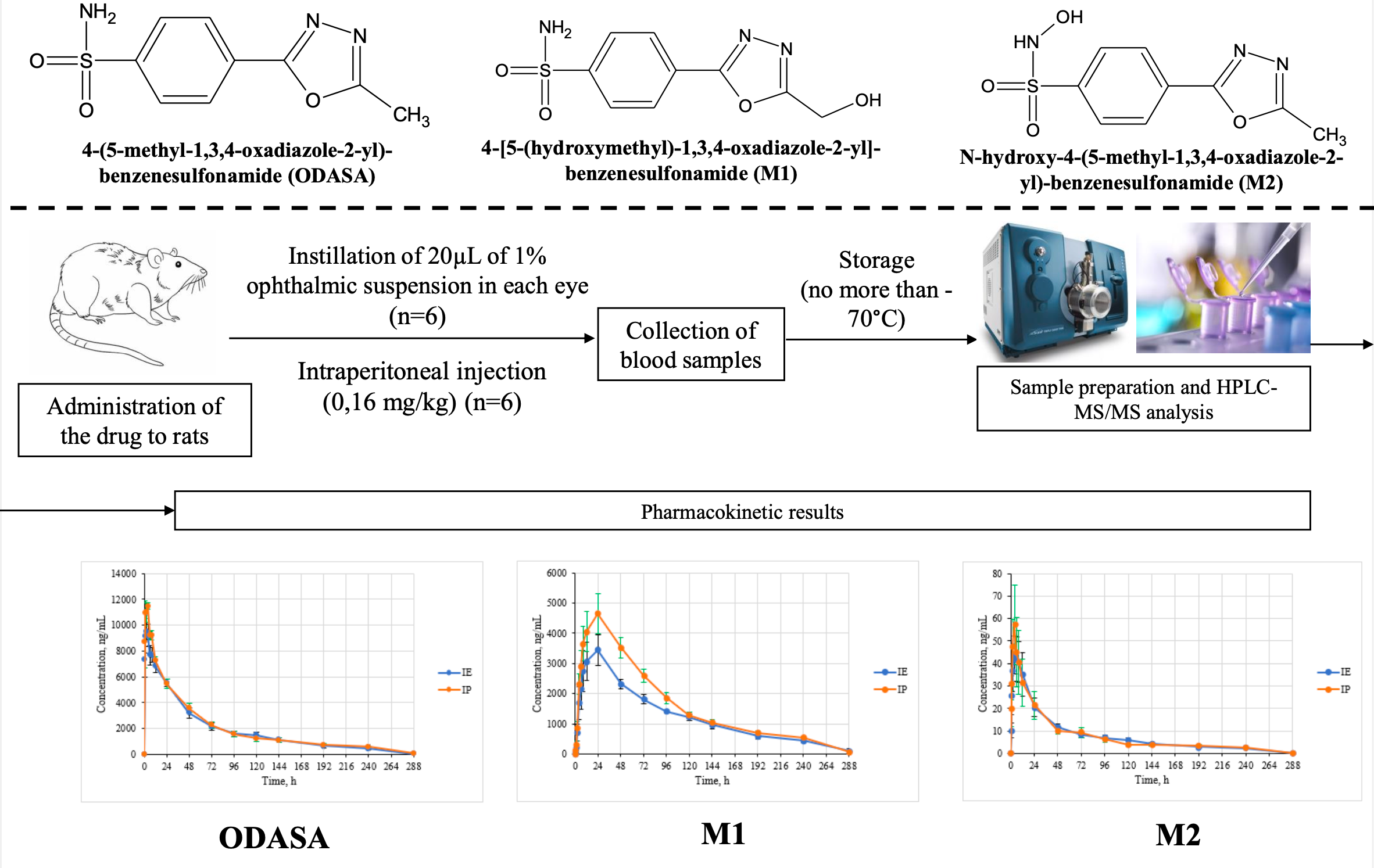
Materials and Methods: The 1% ocular suspension of ODASA was instilled into each eye in a volume of 20 µL to a group of 6 Wistar rats. The drug was also administrated by intraperitoneal injection at a dose of 1.6 mg/kg to another group of 6 Wistar rats. Sampling was carried out before administration and at 16 points for 288 hours after administration. HPLC-MS/MS was used for quantification of ODASA and its metabolites 4-[5-(hydroxymethyl)-1,3,4-oxadiazole-2-yl]-benzenesulfonamide (M1) and N-hydroxy-4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonamide (M2).
Results: The high concentration level of ODASA and M1 was observed: maximum blood concentration (Cmax) of ODASA after eye instillation was 10326±532 ng/mL, Cmax of M1 was 3722±505 ng/mL(M±SEM). The Cmax value of M2 was lower – 49.9±9.4 ng/mL (M±SEM). The half-life time of the studied compounds was more than 69 h. M1 has the greatest affinity for red blood cells. The value of the area under the “concentration-time” curve (AUC0-t) of M1 in bloodwas more than 150 times higher than AUC0-t of M1 in plasma.
Conclusion: The ODASA and its metabolites have long half-life time and high exposition in blood in comparison with that in plasma, which proves their accumulation in red blood cells.
Graphical Abstract
Keywords:
HPLC-MS/MS, whole blood, validation, pharmacokinetics, bioavailability, rats, carbonic anhydrase II inhibitor, N-hydroxysulfonamideReferences
Begou O, Drabert K, Theodoridis G, Tsikas D (2020) GC-NICI-MS analysis of acetazolamide and other sulfonamide (R-SO2-NH2) drugs as pentafluorobenzyl derivatives [R-SO2-N(PFB)2] and quantification of pharmacological acetazolamide in human urine. Journal of Pharmaceutical Analysis 10(1): 49–59. https://doi.org/10.1016/j.jpha.2019.11.006 [PubMed] [PMC]
Busardo FP, Lo Faro AF, Sirignano A, Giorgetti R, Carlier J (2022) In silico, in vitro, and in vivo human metabolism of acetazolamide, a carbonic anhydrase inhibitor and common “diuretic and masking agent” in doping. Archives of Toxicology 96(7): 1989–2001. https://doi.org/10.1007/s00204-022-03289-z [PubMed] [PMC]
Dhandar AG, Chaudhari SR, Ganorkar SB, Patil AS, Surana SJ (2022) Mini-review on bioanalytical estimation of brinzolamide. Current Pharmaceutical Analysis 18(3): 265–272. https://doi.org/10.2174/1573412917666210812103414
ICH guideline M10 on bioanalytical method validation and study sample analysis (2022) https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m10-bioanalytical-method-validation-step-5_en.pdf.
Khokhlov AL, Shetnev AA, Korsakov MK, Fedorov VN, Tyushina AN, Volkhin NN, Vdovichenko VP (2023) Pharmacological properties of sulfonamide derivatives — new inhibitors of carbonic anhydrase. Bulletin of Experimental Biology and Medicine 175 (2): 166–170. https://doi.org/10.47056/0365-9615-2023-175-2-166-170
Khokhlov AL, Yaichkov II, Shetnev AА, Korsakov MK, Volkhin NN, Petukhov SS, Tyushina AN, Lasaryanz OE (2024) The evaluation of pharmacokinetic parameters of 4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonamide and its metabolites in rat plasma. Research Results in Pharmacology 10(4): 67–76. https://doi.org/10.18413/rrpharmacology.10.523
Kurysheva NI (2020) Carbonic anhydrase inhibitors in the treatment of glaucoma. Review. Part II. Ophthalmology in Russia [Oftal'mologiya] 17(4): 676–682. https://doi.org/10.18008/1816-5095-2020-4-676-682 [in Russian]
Lo Faro AF, Tini A, Gottardi M, Pirani F, Sirignano A, Giorgetti R, Busardò FP (2021) Development and validation of a fast ultra-high-performance liquid chromatography tandem mass spectrometry method for determining carbonic anhydrase inhibitors and their metabolites in urine and hair. Drug Testing and Analysis 13(8): 1552–1560. https://doi.org/10.1002/dta.3055 [PubMed] [PMC]
Mironov AN (ed.) (2012) Guidelines for conducting preclinical studies of medicines. Volume 1. Polygraph Plus, Moscow, 944 pp.
Naageshwaran V, Ranta V-P, GumG, Bhoopathy S, Urtti A, Del Amo EM (2021) Comprehensive ocular and systemic pharmacokinetics of brinzolamide in rabbits after intracameral. Topical, and Intravenous Administration. Journal of Pharmaceutical Sciences 110(1): 529–535. https://doi.org/10.1016/j.xphs.2020.09.051 [PubMed]
On Approval of the Rules for Conducting Bioequivalence Studies on Medicines in the Eurasian Economic Union. Decision of the Council of the Eurasian Economic Commission № 85 of November 3, 2016 № 85 (2016) http://docs.cntd.ru/document/456026107(access date: 16.10.2024).
Yaichkov II, Khokhlov AL, Korsakov MK, Shetnev AA, Volkhin NN, Petukhov SS (2024) Pharmacokinetics study of a new isoxazole derivative in rats using HPLC-MS/MS for blood sample analysis. Regulatory Research and Medicine Evaluation 14(3): 304–316. https://doi.org/10.30895/1991-2919-2024-14-3-304-316
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Khokhlov AL, Yaichkov II, Shetnev AA, Korsakov MK, Volkhin NN, Petukhov SS, Tyushina AN, Lasaryanz OE

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

