Salvia extracts: Unraveling phenolic compounds and assessing their antiglycation, anti-inflammatory, and cytotoxic properties
DOI:
https://doi.org/10.18413/rrpharmacology.11.534Abstract
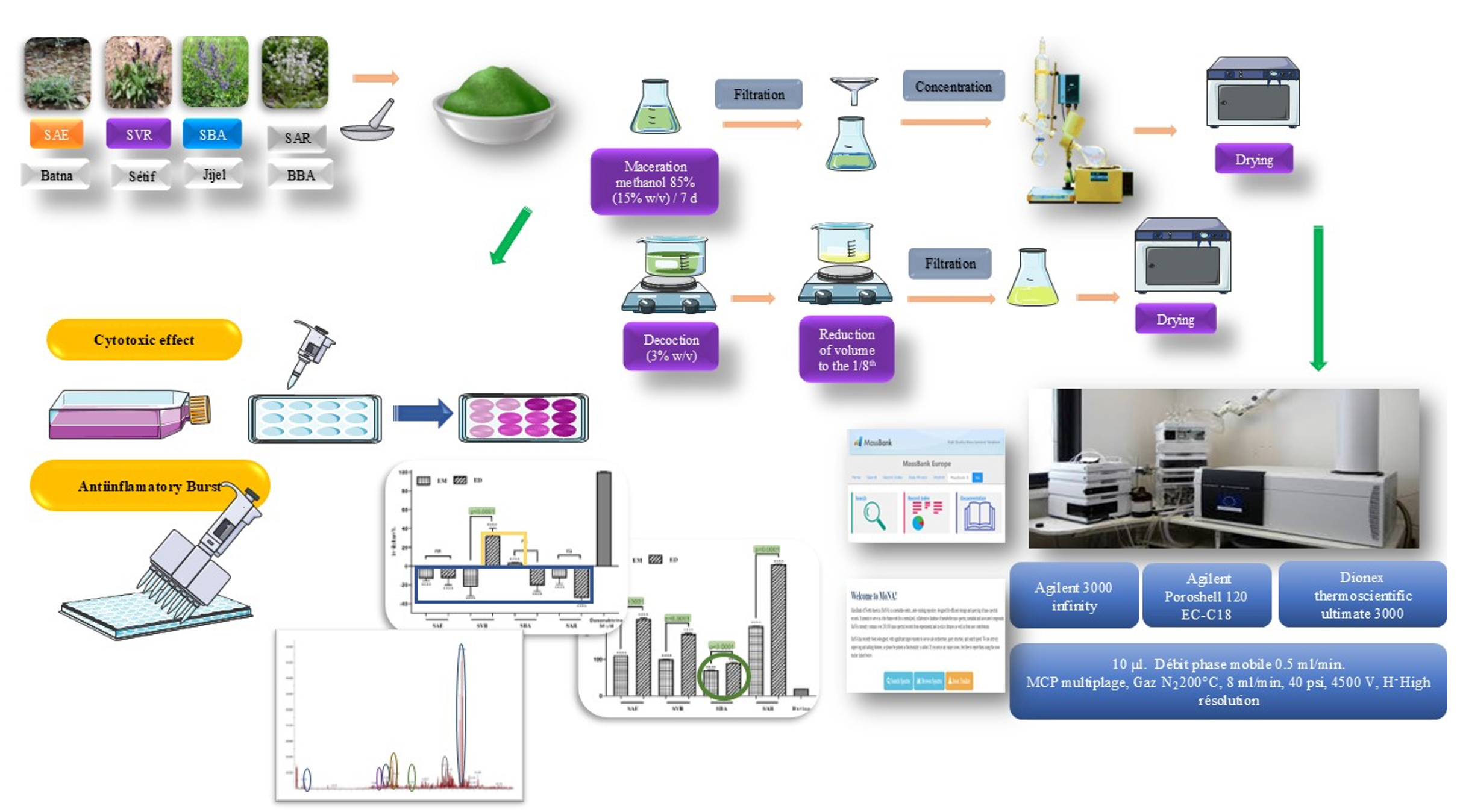
Introduction: This study aimed to identify compounds present in the methanolic extract (ME) of four Salvia species S. aegyptiaca (SAE), S. verbenaca (SVE), S. barrelieri (SBA), and S. argentea (SAR), using HPLC ESI-QTOF MS/MS, and to evaluate their antiglycation, anti-inflammatory, and cytotoxic effects for both methanolic and decoction extracts (DE). The research focused on exploring the phytochemical profile and biological activities of Salvia species, which are known for their medicinal properties.
Materials and Methods: HPLC ESI-QTOF MS/MS was employed to identify phenolic compounds in the extracts. Antiglycation activity was assessed using a model system, while cytotoxicity was evaluated using two cell lines: mouse fibroblast cells (3T3) and human cervical cancer cells (HeLa).
Results: Four major phenolic compounds were identified in all four plants: caffeoyl-O-hexoside(glucoside), rosmarinic acid, derivatives of apigenin, and an isomer of p-coumaroylquinic acid. Additionally, luteolin-7-O-glucoside was detected in all extracts except SAE. All extracts demonstrated significant antiglycation efficacy, with inhibition efficiency exceeding 69% at 2 mg/mL. Notably, the methanolic extract of S. barrelieri (ME SBA) exhibited the highest activity, achieving an IC50 of approximately 35 µg/mL. Cytotoxicity testing revealed weak and insignificant effects for decoction extracts on 3T3 cells, whereas slight proliferation was observed with methanolic extracts. Similarly, most extracts showed no toxicity toward HeLa cells, except for the decoction extract of S. verbenaca (ED SVR), which exhibited some cytotoxicity.
Discussion: The presence of common phenolic compounds across the studied Salvia species highlights their potential as sources of bioactive molecules. The observed antiglycation activity suggests these extracts could be beneficial in preventing glycation-related diseases. However, the cytotoxicity results indicate that further optimization may be required to enhance their therapeutic potential.
Conclusion: This study successfully identified key compounds in Salvia species and demonstrated their notable antiglycation properties. While cytotoxic effects were minimal, the findings underscore the potential of these plants as natural remedies for specific health conditions, warranting further investigation into their pharmacological applications.
Graphical Abstract
Keywords:
Salvia species, AGE inhibition, Anti-inflammatory, cytotoxic effect, HPLC-ESI-QTOFReferences
Abdallah Q, Al-Deeb I, Bader A, Hamam F, Saleh K, Abdulmajid A (2018) Anti-angiogenic activity of Middle East medicinal plants of the Lamiaceae family. Molecular Medicine Reports 18(2): 2441–2448. https://doi.org/10.3892/mmr.2018.9155 [PubMed] [PMC]
Abu-Dahab R, Afifi F, Kasabri V, Majdalawi L, Naffa R (2012) Comparison of the antiproliferative activity of crude ethanol extracts of nine salvia species grown in Jordan against breast cancer cell line models. Pharmacognosy Magazine 8(32): 319–324. https://doi.org/10.4103/0973-1296.103664 [PubMed] [PMC]
Afonso AF, Pereira OR, Válega M, Silva A, Cardoso SM (2018) Metabolites and biological activities of Thymus zygis, Thymus pulegioides, and Thymus fragrantissimus grown under organic cultivation. Molecules 23(7): 1514. https://doi.org/10.3390/molecules23071514[PubMed] [PMC]
Agawane SB, Gupta VS, Kulkarni MJ, Bhattacharya AK, Koratkar SS (2019) Chemo-biological evaluation of antidiabetic activity of Mentha arvensis L. and its role in inhibition of advanced glycation end products. Journal of Ayurveda and Integrative Medicine 10(3): 166–170. https://doi.org/10.1016/j.jaim.2017.07.003 [PubMed] [PMC]
Al-Barazanjy RK, Dizaye K, Al-Asadye AA (2013) Cytotoxic and cytogenetic effects of Salvia officinalis on different tumor cell lines. Middle East Journal of Internal Medicine 63: 1–11.
Amira S, Dade M, Schinella G, Ríos J-L (2012) Anti-inflammatory, anti-oxidant, and apoptotic activities of four plant species used in folk medicine in the Mediterranean basin. Pakistan Journal of Pharmaceutical Sciences 25(1): 65–72. [PubMed]
Ayatollahi AM, Ghanadian M, Att-Ur-Rahman R, Mesaik MA, Khalid AS, Adeli F (2015) Methoxylated flavones from Salvia Mirzayanii Rech. f. and Esfand with immunosuppressive properties. Iranian Journal of Pharmaceutical Research 14(3): 955–960. [PubMed] [PMC]
Baeza G, Sarriá B, Bravo L, Mateos R (2016) Exhaustive qualitative LC-DAD-MS(n) analysis of Arabica green coffee beans: Cinnamoyl-glycosides and Cinnamoylshikimic Acids as New Polyphenols in Green Coffee. Journal of Agricultural and Food Chemistry 64(51): 9663–9674. https://doi.org/10.1021/acs.jafc.6b04022 [PubMed]
Bechkri S, Alabdul Magid A, Voutquenne-Nazabadioko L, Berrehal D, Kabouche A, Lehbili M, Lakhal H, Abedini A, Gangloff SC, Morjani H, Kabouche Z (2019) Triterpenes from Salvia argentea var. aurasiaca and their antibacterial and cytotoxic activities. Fitoterapia 139: 104296. https://doi.org/10.1016/j.fitote.2019.104296 [PubMed]
Behroozi J, Divsalar A, Saboury AA (2014) Honey bee venom decreases the complications of diabetes by preventing hemoglobin glycation. Journal of Molecular Liquids 199: 371–375. https://doi.org/10.1016/j.molliq.2014.09.034
BenKhedher MR, Hafsa J, Haddad M, Hammami M (2020) Inhibition of protein glycation by combined antioxidant and antiglycation constituents from a phenolic fraction of Sage (Salvia officinalis L.). Plant Foods for Human Nutrition 75(4): 505–511. https://doi.org/10.1007/s11130-020-00838-8 [PubMed]
BenSaid R, Hamed AI, Mahalel UA, Al-Ayed AS, Kowalczyk M, Moldoch J, Oleszek W, Stochmal A (2017) Tentative characterization of polyphenolic compounds in the male flowers of Phoenix dactylifera by liquid chromatography coupled with mass spectrometry and DFT. International Journal of Molecular Sciences 18(3): 512. https://doi.org/10.3390/ijms18030512 [PubMed] [PMC]
Benarba B (2016) Medicinal plants used by traditional healers from South-West Algeria: An ethnobotanical study. Journal of Intercultural Ethnopharmacology 5(4): 320–330. https://doi.org/10.5455/jice.20160814115725 [PubMed] [PMC]
Brito A, Ramirez JE, Areche C, Sepúlveda B, Simirgiotis MJ (2014) HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules 19(11): 17400–17421. https://doi.org/10.3390/molecules191117400 [PubMed] [PMC]
Chinchansure AA, Korwar AM, Kulkarni MJ, Joshi SP (2015) Recent development of plant products with anti-glycation activity: a review. RSC Advances 5: 31113–31138. https://doi.org/10.1039/C4RA14211J
Clifford MN, Marks S, Knight S, Kuhnert N (2006) Characterization by LC-MSn of four new classes of p-coumaric acid-containing diacyl chlorogenic acids in green coffee beans. Journal of Agricultural and Food Chemistry 54(12): 4095–4101. https://doi.org/10.1021/jf060536p [PubMed]
Dehkordi FJ, Kharazian N, Lorigooini Z (2020) Characterization of flavonoid components in Scutellaria L. species (Lamiaceae) using finger-printing analysis. Acta Biologica Cracoviensia Series Botanica 62(1): 79–96. https://doi.org/0.24425/abcsb.2020.131666
Deo P, Hewawasam E, Karakoulakis A, Claudie DJ, Nelson R, Simpson BS, Smith NM, Semple SJ (2016) In vitro inhibitory activities of selected Australian medicinal plant extracts against protein glycation, angiotensin converting enzyme (ACE) and digestive enzymes linked to type II diabetes. BMC Complementary and Alternative Medicine 16(1): 435. https://doi.org/10.1186/s12906-016-1421-5 [PubMed] [PMC]
El-Seedi HR, Burman R, Mansour A, Turki Z, Boulos L, Gullbo J, Göransson U (2013) The traditional medical uses and cytotoxic activities of sixty-one Egyptian plants: Discovery of an active cardiac glycoside from Urginea maritima. Journal of Ethnopharmacology 145(3): 746–757. https://doi.org/10.1016/j.jep.2012.12.007 [PubMed]
Erdemoglu N, Turan NN, Cakõcõ I, Sener B, Aydõn A (2006) Antioxidant activities of some Lamiaceae plant extracts. Phytotherapy Research 20(1): 9–13. https://doi.org/10.1002/ptr.1816 [PubMed]
Esmaeili MA, Kanani MR, Sonboli ALI (2010) Salvia reuterana extract prevents formation of advanced glycation end products: An in vitro study. Iranian Journal of Pharmaceutical Sciences 6: 33–50.
Firuzi O, Miri R, Asadollahi M, Eslami S, Jassbi AR (2013) Cytotoxic, antioxidant and antimicrobial activities and phenolic contents of eleven Salvia species from Iran. Iranian Journal of Pharmaceutical Research 12(4): 801. [PubMed] [PMC]
Govindaraj J, Sorimuthu Pillai S (2015) Rosmarinic acid modulates the antioxidant status and protects pancreatic tissues from glucolipotoxicity mediated oxidative stress in high-fat diet: streptozotocin-induced diabetic rats. Molecular and Cellular Biochemistry 404(1–2): 143–159. https://doi.org/10.1007/s11010-015-2374-6 [PubMed]
Guaouguaou F-E, Bebaha MAA, Taghzouti K, Bouyahya A, Bakri Y, Dakka N, Es-Safi NE (2018) Cytotoxicological investigation of the essential oil and the extracts of Cotula cinereaand Salvia verbenaca from Morocco. BioMed Research International 2018: https://doi.org/10.1155/2018/7163961 [PubMed] [PMC]
Helfand SL, Werkmeister J, Roder JC (1982) Chemiluminescence response of human natural killer cells. I. The relationship between target cell binding, chemiluminescence, and cytolysis. The Journal of Experimental Medicine 156(2): 492–505. https://doi.org/10.1084/jem.156.2.492 [PubMed] [PMC]
Hossain MB, Rai DK, Brunton NP, Martin-Diana AB, Barry-Ryan C (2010) Characterization of phenolic composition in Lamiaceae spices by LC-ESI-MS/MS. Journal of Agricultural and Food Chemistry 58(19): 10576–10581. https://doi.org/10.1021/jf102042g [PubMed]
Imanshahidi M, Hosseinzadeh H (2006) The pharmacological effects of Salvia species on the central nervous system. Phytotherapy Research 20(6): 427–437. https://doi.org/10.1002/ptr.1898 [PubMed]
Janicsák G, Zupkó I, Nikolova MT, Forgo P, Vasas A, Máthé I, Blunden G, Hohmann J (2011) Bioactivity-guided study of antiproliferative activities of Salvia extracts. Natural Product Communications 6(5): 1934578X1100600501. https://doi.org/10.1177/1934578X1100600501 [PubMed]
Jantan I, Harun NH, Septama AW, Murad S, Mesaik MA (2011) Inhibition of chemiluminescence and chemotactic activity of phagocytes in vitro by the extracts of selected medicinal plants. Journal of Natural Medicines 65(2): 400–405. https://doi.org/10.1007/s11418-010-0492-8 [PubMed]
Jean D, Pouligon M, Dalle C (2015) Evaluation in vitro of AGE-crosslinks breaking ability of rosmarinic acid. Glycative Stress Research 2: 204–207.
Jiang D-x, Liu S-r, Zhang M-h, Zhang T, Ma W-j, Mu X, Chen W (2015) Luteolin prevents fMLP-induced neutrophils adhesion via suppression of LFA-1 and phosphodiesterase 4 activity. Journal of Integrative Agriculture 14: 140–147. https://doi.org/10.1016/S2095-3119(14)60904-7
Kamatou GPP, Van Zyl RL, Davids H, Van Heerden FR, Lourens ACU, Viljoen AM (2008) Antimalarial and anticancer activities of selected South African Salvia species and isolated compounds from radula. South African Journal of Botany 74(2): 238–243. https://doi.org/10.1016/j.sajb.2007.08.001
Katanić Stanković JS, Srećković N, Mišić D, Gašić U, Imbimbo P, Monti DM, Mihailović V (2020) Bioactivity, biocompatibility and phytochemical assessment of lilac sage, Salvia verticillata L. (Lamiaceae) – A plant rich in rosmarinic acid. Industrial Crops and Products 143: 111932. https://doi.org/10.1016/j.indcrop.2019.111932
Keshavarz M, Mostafaie A, Mansouri K, Bidmeshkipour A, Motlagh HRM, Parvaneh S (2010) In vitro and ex vivo antiangiogenic activity of Salvia officinalis. Phytotherapy research 24(10): 1526–1531. https://doi.org/10.1002/ptr.3168 [PubMed]
Kontogianni VG, Tomic G, Nikolic I, Nerantzaki AA, Sayyad N, Stosic-Grujicic S, Stojanovic I, Gerothanassis IP, Tzakos AG (2013) Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chemistry 136(1): 120–129. https://doi.org/10.1016/j.foodchem.2012.07.091[PubMed]
Koutsoulas A, Čarnecká M, Slanina J, Tóth J, Slaninová I (2019) Characterization of phenolic compounds and antiproliferative effects of Salvia pomifera and Salvia fruticosa extracts. Molecules 24(16): 2921. https://doi.org/10.3390/molecules24162921 [PubMed] [PMC]
Kumar S, Singh A, Kumar B (2017) Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. Journal of Pharmaceutical Analysis 7(4): 214–222. https://doi.org/10.1016/j.jpha.2017.01.005 [PubMed] [PMC]
Lehbili M, Alabdul Magid A, Kabouche A, Voutquenne-Nazabadioko L, Abedini A, Morjani H, Gangloff SC, Kabouche Z (2018) Antibacterial, antioxidant and cytotoxic activities of triterpenes and flavonoids from the aerial parts of Salvia barrelieri Etl. Natural Product Research 32(22): 2683–2691. https://doi.org/10.1080/14786419.2017.1378207[PubMed]
Li J, Guo W-J, Yang Q-Y (2002) Effects of ursolic acid and oleanolic acid on human colon carcinoma cell line HCT15. World Journal of Gastroenterology 8(3): 493–495. https://doi.org/10.3748/wjg.v8.i3.493 [PubMed] [PMC]
Li S, Lin Z, Jiang H, Tong L, Wang H, Chen S (2016) Rapid identification and assignation of the active ingredients in fufang banbianlian injection using HPLC-DAD-ESI-IT-TOF-MS. Journal of Chromatographic Science 54(7): 1225–1237. https://doi.org/10.1093/chromsci/bmw055 [PubMed]
Lima CF, Valentao PCR, Andrade PB, Seabra RM, Fernandes-Ferreira M, Pereira-Wilson C (2007) Water and methanolic extracts of Salvia officinalis protect HepG2 cells from t-BHP induced oxidative damage. Chemico-Biological Interactions 167(2): 107–115. https://doi.org/10.1016/j.cbi.2007.01.020 [PubMed]
Mahomoodally F, Aumeeruddy-Elalfi Z, Venugopala KN, Hosenally M (2019) Antiglycation, comparative antioxidant potential, phenolic content and yield variation of essential oils from 19 exotic and endemic medicinal plants. Saudi Journal of Biological Sciences 26(7): 1779–1788. https://doi.org/10.1016/j.sjbs.2018.05.002 [PubMed] [PMC]
Mamache W, Amira S, Ben Souici C, Laouer H, Benchikh F (2020) In vitro antioxidant, anticholinesterases, anti‐α‐amylase, and anti‐α‐glucosidase effects of Algerian Salvia aegyptiaca and Salvia verbenaca. Journal of Food Biochemistry 44(11): e13472. https://doi.org/10.1111/jfbc.13472 [PubMed]
Markham KR (1982) Techniques of flavonoid identification. Academic Press, London, 113 pp.
Matsuda H, Wang T, Managi H, Yoshikawa M (2003) Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities. Bioorganic & Medicinal Chemistry 11(24): 5317–5323. https://doi.org/10.1016/j.bmc.2003.09.045 [PubMed]
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods 65(1–2): 55–63. https://doi.org/10.1016/0022-1759(83)90303-4 [PubMed]
Oliveira-Alves SC, Vendramini-Costa DB, Betim Cazarin CB, Maróstica Júnior MR, Borges Ferreira JP, Silva AB, Prado MA, Bronze MR (2017) Characterization of phenolic compounds in chia (Salvia hispanica L.) seeds, fiber flour and oil. Food Chemistry 232: 295–305. https://doi.org/10.1016/j.foodchem.2017.04.002 [PubMed]
Ou J, Huang J, Wang M, Ou S (2017) Effect of rosmarinic acid and carnosic acid on AGEs formation in vitro. Food Chemistry 221: 1057–1061. https://doi.org/10.1016/j.foodchem.2016.11.056 [PubMed]
Ou J, Huang J, Zhao D, Du B, Wang M (2018) Protective effect of rosmarinic acid and carnosic acid against streptozotocin-induced oxidation, glycation, inflammation and microbiota imbalance in diabetic rats. Food & Function 9(2): 851–860. https://doi.org/10.1039/C7FO01508A [PubMed]
Perera N, Soysa P, Abeytunga T, Ramesha R (2008) Antioxidant and cytotoxic properties of three traditional decoctions used for the treatment of cancer in Sri Lanka. Pharmacognosy Magazine 4(15): 172–181.
Popov AM, Osipov AN, Korepanova EA, Krivoshapko ON, Artiukov AA (2013) Study of antioxidant and membrane activity of rosmarinic acid using different model systems. Molecular Biophysics 58: 775–785. https://doi.org/10.1134/S0006350913050126[PubMed] [in Russian]
Prastya ME, Astuti RI, Batubara I, Wahyudi AT (2019) Antioxidant, antiglycation and in vivo antiaging effects of metabolite extracts from marine sponge-associated bacteria. Indian Journal of Pharmaceutical Sciences 81: 344–353.
Rahbar S, Figarola JL (2002) Inhibitors and breakers of advanced glycation endproducts (AGEs): a review. Current Medicinal Chemistry-Immunology, Endocrine & Metabolic Agents 2: 135–161. https://doi.org/10.2174/1568013023358889
Revoltella S, Baraldo G, Waltenberger B, Schwaiger S, Kofler P, Moesslacher J, Huber-Seidel A, Pagitz K, Kohl R, Jansen-Duerr P, Stuppner H (2018) Identification of the NADPH oxidase 4 inhibiting principle of Lycopus europaeus. Molecules 23(3): 635. [PubMed] [PMC]
Ribeiro D, Freitas M, Tomé SM, Silva AMS, Porto G, Fernandes E (2013) Modulation of human neutrophils’ oxidative burst by flavonoids. European Journal of Medicinal Chemistry 67: 280–292. https://doi.org/10.1016/j.ejmech.2013.06.019 [PubMed]
Rocha J, Eduardo-Figueira M, Barateiro A, Fernandes A, Brites D, Bronze R, Duarte CMM, Serra AT, Pinto R, Freitas M, Fernandes E, Silva-Lima B, Mota-Filipe H, Sepodes B (2015) Anti-inflammatory effect of rosmarinic acid and an extract of Rosmarinus officinalis in rat models of local and systemic inflammation. Basic & Clinical Pharmacology & Toxicology 116(5): 398–413. https://doi.org/10.1111/bcpt.12335 [PubMed]
Safari MR, Azizi O, Heidary SS, Kheiripour N, Ravan AP (2018) Antiglycation and antioxidant activity of four Iranian medical plant extracts. Journal of Pharmacopuncture 21(2): 82–89. https://doi.org/10.3831/KPI.2018.21.010 [PubMed] [PMC]
Sheng Z, Ai B, Zheng L, Zheng X, Xu Z, Shen Y, Jin Z (2018) Inhibitory activities of kaempferol, galangin, carnosic acid and polydatin against glycation and α-amylase and α-glucosidase enzymes. International Journal of Food Science & Technology 53: 755–766. https://doi.org/10.1111/ijfs.13579
Soomro S, Sangi S, Mashooq AA (2019) In vitro biological activity of ethanolic extract of Maramiyah (Salvia Libanotica) and its combination with essential oil. International Journal of Pharmaceutical and Phytopharmacological Research 9: 32–37.
Šulniūtė V, Pukalskas A, Venskutonis PR (2017) Phytochemical composition of fractions isolated from ten Salvia species by supercritical carbon dioxide and pressurized liquid extraction methods. Food Chemistry 224: 37–47. https://doi.org/10.1016/j.foodchem.2016.12.047 [PubMed]
Tang J, Dunshea FR, Suleria HAR (2020) LC-ESI-QTOF/MS characterization of phenolic compounds from medicinal plants (hops and juniper berries) and their antioxidant activity. Foods 9(1): 7. https://doi.org/10.3390/foods9010007 [PubMed]
Tao L, Xu M, Dai X, Ni T, Li D, Jin F, Wang H, Tao L, Pan B, Woodgett JR, Qian Y, Liu Y (2018) Polypharmacological profiles underlying the antitumor property of Salvia miltiorrhiza root (danshen) interfering with NOX-dependent neutrophil extracellular traps. Oxidative Medicine and Cellular Longevity 2018(1): 4908328–4908328. https://doi.org/10.1155/2018/4908328 [PubMed] [PMC]
Tohamy AA, El-Garawani IM, Ibrahim SR, Moneim AEA (2016) The apoptotic properties of Salvia aegyptiaca and Trigonella foenum-graecum extracts on Ehrlich ascites carcinoma cells: the effectiveness of combined treatment. Research Journal of Pharmaceutical Biological and Chemical Sciences 7: 1872–1883.
Toplan G, Gizem, Kurkcuoglu M, Goger F, İşcan G, Ağalar HG, Mat A, Baser KHC, Koyuncu M, Sarıyar G (2017) Composition and biological activities of Salvia veneris Hedge growing in Cyprus. Industrial Crops and Products 97: 41–48. https://doi.org/10.1016/j.indcrop.2016.11.055
Tusi SK, Khodagholi F (2014) Salvia macilenta exhibits antiglycating activity and protects PC12 cells against H2O2-induced apoptosis. Cytotechnology 66(1): 169–179. https://doi.org/10.1007/s10616-013-9550-x [PubMed] [PMC]
Ul Haq F, Ali A, Akhtar N, Aziz N, Khan MN, Ahmad M, Musharraf SG (2020) A high-throughput method for dereplication and assessment of metabolite distribution in Salvia species using LC-MS/MS. Journal of Advanced Research 24: 79–90. https://doi.org/10.1016/j.jare.2020.02.001 [PubMed] [PMC]
Winterbourn CC, Kettle AJ, Hampton MB (2016) Reactive oxygen species and neutrophil function. Annual Review of Biochemistry 85: 765–792. [PubMed]
Xavier CPRF, Lima C, Fernandes-Ferreira M, Pereira-Wilson C (2008) Induction of apoptosis and inhibition of proliferation in colon cancer cells by Salvia fruticosa, Salvia officinalis and rosmarinic acid. Planta Medica 74: PA19.
Xavier JdA, Valentim IB, Camatari FOS, de Almeida AMM, Goulart HF, Ferro JNdS, Barreto EdO, Cavalcanti BC, Bottoli CBG, Goulart MOF (2017) Polyphenol profile by UHPLC-MS/MS, anti-glycation, antioxidant and cytotoxic activities of several samples of propolis from the northeastern semi-arid region of Brazil. Pharmaceutical Biology 55(1): 1884–1893. https://doi.org/10.1080/13880209.2017.1340962 [PubMed] [PMC]
Yang S-C, Chen P-J, Chang S-H, Weng Y-T, Chang F-R, Chang K-Y, Chen C-Y, Kao T-I, Hwang T-L (2018) Luteolin attenuates neutrophilic oxidative stress and inflammatory arthritis by inhibiting Raf1 activity. Biochemical Pharmacology 154: 384–396. https://doi.org/10.1016/j.bcp.2018.06.003 [PubMed]
Yang ST, Wu X, Rui W, Guo J, Feng YF (2015) UPLC/Q-TOF-MS Analysis for identification of hydrophilic phenolics and lipophilic diterpenoids from Radix Salviae Miltiorrhizae. Acta Chromatographica 27: 711–728. https://doi.org/10.1556/achrom.27.2015.4.9
Yap H-YY, Tan N-H, Ng S-T, Tan C-S, Fung S-Y (2018) Inhibition of protein glycation by tiger milk mushroom [Lignosus Rhinocerus (Cooke) Ryvarden] and search for potential anti-diabetic activity-related metabolic pathways by genomic and transcriptomic data mining. Frontiers in Pharmacology 9: 103. https://doi.org/10.3389/fphar.2018.00103 [PubMed] [PMC]
Zhang Y, Xiong H, Xu X, Xue X, Liu M, Xu S, Liu H, Gao Y, Zhang H, Li X (2018) Compounds identification in semen cuscutae by ultra-high-performance liquid chromatography (UPLCs) coupled to electrospray ionization mass spectrometry. Molecules (Basel, Switzerland) 23(5): 1199. https://doi.org/10.3390/molecules23051199 [PubMed] [PMC]
Zhou H, Fu B, Xu B, Mi X, Li G, Ma C, Xie J, Li J, Wang Z (2017) Rosmarinic acid alleviates the endothelial dysfunction induced by hydrogen peroxide in rat aortic rings via activation of AMPK. Oxidative Medicine and Cellular Longevity 2017: 7091904. https://doi.org/10.1155/2017/7091904 [PubMed] [PMC]
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Walid Mamache, Amor Bencheikh, Abderrahim Benslama, Fatima Bencheikh, Hassiba Benabdallah, Smain Amira

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

