Improving antitumor targeting via using PL3 homing peptide and cell-penetrating peptide
DOI:
https://doi.org/10.18413/rrpharmacology.9.10028Abstract
Introduction: Tumor-homing peptides have gained great attention as tools for the development of non-invasive and targeting drug delivery systems (DDS) to minimize drug systemic toxicity and enhance bioavailability. This study aims to improve antitumor targeting in prostate cancer via uploading a drug to a DDS comprised of a cell penetrating peptide decorated with a tumor-homing peptide, PL3.
Material and Methods: The DDS was constructed via solid-phase peptide synthesis and then characterized via mass spectrum and high performance liquid chromatography. A cell viability assessment to evaluate its cytotoxicity on both tumor (prostate cancer cells) and normal cells was conducted, while a confocal laser scanning microscope and flow-cytometer were employed to investigate internalization. To inspect the effectiveness of the drug-loaded DDS, a biochemical enzyme inhibition assay on the target enzyme dihydrofolate reductase (DHFR) was performed.
Results and Discussion: The findings supported the succeeded synthesis and loading of the drug into this carrier system and demonstrated its high efficacy in cytotoxic effect and inhibiting DHFR with considerable cellular uptake in prostate cancer cells.
Conclusion: The drug was delivered to the target prostate cancer cells by the PL3-functionalized DDS, limiting its localization to tumor cells rather than normal cells. Therefore, the study results highlighted the significance of the DDS in tumor therapy interventions.
Graphical Abstact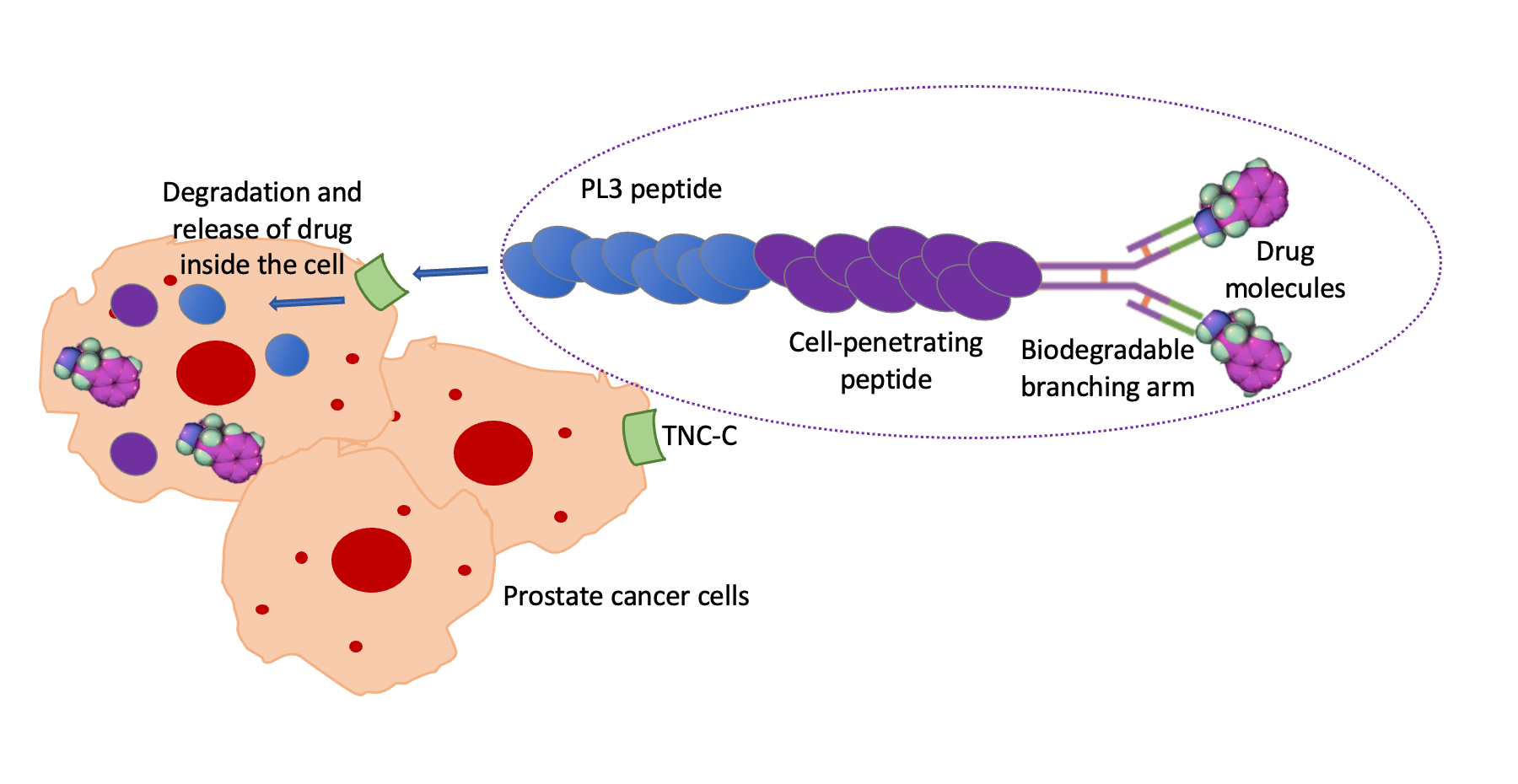
Keywords:
cancer chemotherapy, cell-penetrating peptide, drug delivery system, prostate cancer, tumor-homing peptideReferences
Al-azzawi S, Masheta D (2019) Designing a drug delivery system for improved tumor treatment and targeting by functionalization of a cell-penetrating peptide. Journal of Pharmaceutical Investigation 49: 643–654.
https://doi.org/10.1007/s40005-018-00424-w
Al-Azzawi S, Masheta D, Guildford A, Phillips G, Santin M (2019) Designing and characterization of a novel delivery system for improved cellular uptake by brain using dendronised Apo-E-derived peptide. Frontiers in Bioengineering and Biotechnology 7: 49. https://doi.org/10.3389/fbioe.2019.00049 [PubMed] [PMC]
Al-Azzawi S, Masheta D, Guildford A, Phillips G, Santin M (2020) A peptide-based nanocarrier for an enhanced delivery and targeting of flurbiprofen into the brain for the treatment of Alzheimer’s disease: An in vitro study. Nanomaterials 10(8): 1590. https://doi.org/10.3390/nano10081590 [PubMed] [PMC]
Al-Azzawi S, Masheta D, Guildford AL, Phillips G, Santin M (2018) Dendrimeric Poly(Epsilon-Lysine) Delivery systems for the enhanced permeability of flurbiprofen across the blood-brain barrier in Alzheimer’s disease. International Journal of Molecular Sciences 19(10): 3224. https://doi.org/10.3390/ijms19103224 [PubMed] [PMC]
Alqosaibi AI (2022) Nanocarriers for anticancer drugs: Challenges and perspectives. Saudi Journal of Biological Sciences 29(6): 103298. https://doi.org/10.1016/j.sjbs.2022.103298 [PubMed] [PMC]
Ayo A, Laakkonen P (2021) Peptide-based strategies for targeted tumor treatment and imaging. Pharmaceutics 13(4): 481. https://doi.org/10.3390/pharmaceutics13040481 [PubMed] [PMC]
Bolhassani A, Jafarzade BS, Mardani G (2017) In vitro and in vivo delivery of therapeutic proteins using cell penetrating peptides. Peptides, 87: 50–63. https://doi.org/10.1016/j.peptides.2016.11.011 [PubMed]
Dissanayake S, Denny WA, Gamage S, Sarojini V (2017) Recent developments in anticancer drug delivery using cell penetrating and tumor targeting peptides. Journal of Controlled Release 250: 62–76. https://doi.org/10.1016/j.jconrel.2017.02.006 [PubMed]
Dubikovskaya EA, Thorne SH, Pillow TH, Contag CH, Wender PA (2008) Overcoming multidrug resistance of small-molecule therapeutics through conjugation with releasable octaarginine transporters. Proceedings of the National Academy of Sciences 105(34): 12128–2133. https://doi.org/10.1073/pnas.0805374105 [PubMed] [PMC]
Dutta B, Barick KC, Hassan PA (2021) Recent advances in active targeting of nanomaterials for anticancer drug delivery. Advances in Colloid and Interface Science 296: 102509. https://doi.org/10.1016/j.cis.2021.102509 [PubMed]
Feng T, Zhao Ya (2017) Nanomaterial-based Drug Delivery Carriers for Cancer Therapy: Clinical Anticancer Drugs for Cancer Treatment, Springer, Singapore, pp. 7–13. https://doi.org/10.1007/978-981-10-3299-8
Gao Q, Feng J, Liu W, Wen C, Wu Y, Liao Q, Zou L, Sui X, Xie T, Zhang J, Hu Y (2022) Opportunities and challenges for co-delivery nanomedicines based on combination of phytochemicals with chemotherapeutic drugs in cancer treatment. Advanced Drug Delivery Reviews 188: 114445. https://doi.org/10.1016/j.addr.2022.114445[PubMed]
Garg NK, Singh B, Jain A, Nirbhavane P, Sharma R, Tyagi RK, Kushwah V, Jain S, Katare OP (2016) Fucose decorated solid-lipid nanocarriers mediate efficient delivery of methotrexate in breast cancer therapeuticsю. Colloids and Surfaces B: Biointerfaces 146: 114–126. https://doi.org/10.1016/j.colsurfb.2016.05.051 [PubMed]
Ghaz-Jahanian MA, Abbaspour-Aghdam F, Anarjan N, Berenjian A, Jafarizadeh-Malmiri H (2015) Application of chitosan-based nanocarriers in tumor-targeted drug delivery. Molecular Biotechnology 57(3): 201–218. https://doi.org/10.1007/s12033-014-9816-3 [PubMed]
Guidotti G, Brambilla L, Rossi D (2017) Cell-penetrating peptides: From basic research to clinics. Trends in Pharmacological Sciences 38(4): 406–424. https://doi.org/10.1016/j.tips.2017.01.003 [PubMed]
Hannoodee M, Meenal M (2022) Methotrexate. in, StatPearls (StatPearls Publishing) [PMC]
Kamalidehghan B, Ghafouri-Fard S, Motevaseli E, Ahmadipour F (2018) Inhibition of human prostate cancer (PC-3) cells and targeting of PC-3-derived prostate cancer stem cells with koenimbin, a natural dietary compound from Murraya koenigii (L) Spreng. Drug Design, Development and Therapy 12: 1119–1133. https://doi.org/10.2147/DDDT.S156826 [PubMed] [PMC]
Kebebe D, Liu Y, Wu Y, Vilakhamxay M, Liu Z, Li J (2018) Tumor-targeting delivery of herb-based drugs with cell-penetrating/tumor-targeting peptide-modified nanocarriers. International Journal of Nanomedicine, 13: 1425–1442. https://doi.org/10.2147/IJN.S156616 [PubMed] [PMC]
Kondo E, Iioka H, Saito K (2021) Tumor‐homing peptide and its utility for advanced cancer medicine. Cancer Science 112(6): 2118–2125. https://doi.org/10.1111/cas.14909 [PubMed] [PMC]
Le Joncour V, Laakkonen P (2018) Seek & destroy, use of targeting peptides for cancer detection and drug delivery. Bioorganic & Medicinal Chemistry 26(10): 2797–2806. https://doi.org/10.1016/j.bmc.2017.08.052 [PubMed]
Lee HL, Dubikovskaya EA, Hwang H, Semyonov AN, Wang H, Jones LR, Twieg RJ, Moerner WE, Wender PA (2008) Single-molecule motions of oligoarginine transporter conjugates on the plasma membrane of Chinese hamster ovary cells. Journal of the American Chemical Society 130(29): 9364–9370. https://doi.org/10.1021/ja710798b [PubMed] [PMC]
Lian B, Wei H, Pan R, Sun J, Zhang B, Wu J, Li X, Tian G (2021) Galactose modified liposomes for effective co-delivery of doxorubicin and combretastatin A4. International Journal of Nanomedicine 16: 457–467. https://doi.org/10.2147/IJN.S283793 [PubMed] [PMC]
Lindgren M, Rosenthal-Aizman K, Saar K, Eiríksdóttir E, Jiang Y, Sassian M, Ostlund P, Hällbrink M, Langel U (2006) Overcoming methotrexate resistance in breast cancer tumour cells by the use of a new cell-penetrating peptide. Biochemical Pharmacology 71(4): 416-425. https://doi.org/10.1016/j.bcp.2005.10.048 [PubMed]
Lingasamy P, Tobi A, Kurm K, Kopanchuk S, Sudakov A, Salumäe M, Rätsep T, Asser T, Bjerkvig R, Teesalu T (2020) Tumor-penetrating peptide for systemic targeting of Tenascin-C. Scientific Reports 10: 1–13. https://doi.org/10.1038/s41598-020-62760-y [PubMed] [PMC]
Litwin MS, Tan HJ (2017) The diagnosis and treatment of prostate cancer: A review. JAMA 317(24): 2532–2542. https://doi.org/10.1001/jama.2017.7248 [PubMed]
Madamsetty VS, Mukherjee A, Mukherjee S (2019) Recent trends of the bio-inspired nanoparticles in cancer theranostics. Frontiers in Pharmacology 10: 1264. https://doi.org/10.3389/fphar.2019.01264 [PubMed] [PMC]
Meikle ST, Perugini V, Guildford AL, Santin M (2011) Synthesis, characterisation and in vitro anti-angiogenic potential of dendron VEGF blockers. Macromolecular Bioscience 11(12): 1761–1765. https://doi.org/10.1002/mabi.201100267 [PubMed]
Merrifield RB (1965) Automated synthesis of peptides. Science 150(3693): 178–185. https://doi.org/10.1126/science.150.3693.178 [PubMed]
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods 65(1-2): 55–63. https://doi.org/10.1016/0022-1759(83)90303-4 [PubMed]
Ramsey JD, Flynn NH (2015) Cell-penetrating peptides transport therapeutics into cells. Pharmacology & Therapeutics 154: 78–86. https://doi.org/10.1016/j.pharmthera.2015.07.003 [PubMed]
Regberg J, Srimanee A, Langel U (2012) Applications of cell-penetrating peptides for tumor targeting and future cancer therapies. Pharmaceuticals (Basel) 5(9): 991–1007. https://doi.org/10.3390/ph5090991 [PubMed]
Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B (2003) Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. The Journal of Biological Chemistry 278(1): 585–590. https://doi.org/10.1074/jbc.M209548200 [PubMed]
Rodríguez H, Suarez M, Albericio F (2010) A convenient microwave-enhanced solid-phase synthesis of short chain N-methyl-rich peptides. Journal of Peptide Science 16(3): 136–140. https://doi.org/10.1002/psc.1209 [PubMed]
Shin MC, Zhang J, Min KA, Lee K, Byun Y, David AE, He H, Yang VC (2014) Cell-penetrating peptides: achievements and challenges in application for cancer treatment. Journal of Biomedical Materials Research. Part A 102(2): 575–587. https://doi.org/10.1002/jbm.a.34859 [PubMed] [PMC]
Silverman R, Holladay M (2014) The organic chemistry of drug design and drug action, Elsevier Inc., USA 517 pp. https://doi/10.1016/C2009-0-64537-2
Soler M, González-Bártulos M, Figueras E, Ribas X, Costas M, Massaguer A, Planas M, Feliu L (2015) Enzyme-triggered delivery of chlorambucil from conjugates based on the cell-penetrating peptide BP16. Organic & Biomolecular Chemistry 13(5): 1470–1480. https://doi.org/10.1039/c4ob01875c [PubMed]
Stanzl EG, Trantow BM, Vargas JR, Wender PA (2013) Fifteen years of cell-penetrating, guanidinium-rich molecular transporters: basic science, research tools, and clinical applications. Accounts of Chemical Research 46(12): 2944–2954. https://doi.org/10.1021/ar4000554 [PubMed] [PMC]
Tang L, Li J, Zhao Q, Pan T, Zhong H, Wang W (2021) Advanced and innovative nano-systems for anticancer targeted drug delivery. Pharmaceutics 13(8): 1151. [PubMed] [PMC]
Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E (2009) C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proceedings of the National Academy of Sciences of the United States of America 106(38): 16157–16162. https://doi.org/10.1073/pnas.0908201106 [PubMed] [PMC]
Torchilin VP, Levchenko TS, Rammohan R, Volodina N, Papahadjopoulos-Sternberg B, D'Souza GG (2003) Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proceedings of the National Academy of Sciences of the United States of America 100(4): 1972–1977. https://doi.org/10.1073/pnas.0435906100[PubMed] [PMC]
Vargas JR, Stanzl EG, Teng NN, Wender PA (2014) Cell-penetrating, guanidinium-rich molecular transporters for overcoming efflux-mediated multidrug resistance. Molecular Pharmaceutics 11(8): 2553–2565.https://doi.org/10.1021/mp500161z [PubMed] [PMC]
Wada A, Terashima T, Kageyama S, Yoshida T, Narita M, Kawauchi A, Kojima H (2019) Efficient prostate cancer therapy with tissue-specific homing peptides identified by advanced phage display technology. Molecular Therapy Oncolytics 12: 138–146. https://doi.org/10.1016/j.omto.2019.01.001 [PubMed] [PMC]
Wender PA, Cooley CB, Geihe EI (2012) Beyond cell penetrating peptides: designed molecular transporters. Drug Discovery Today. Technologies 9(1): e49–e55. https://doi.org/10.1016/j.ddtec.2011.07.004 [PubMed] [PMC]
Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB (2000) The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proceedings of the National Academy of Sciences of the United States of America 97(24): 13003–13008. https://doi.org/10.1073/pnas.97.24.13003 [PubMed] [PMC]
Xu L, Zhang H, Wu Y (2014) Dendrimer advances for the central nervous system delivery of therapeutics. ACS Chemical Neuroscience 5(1): 2–13. https://doi.org/10.1021/cn400182z [PubMed] [PMC]
Yang W, Luo D, Wang S, Wang R, Chen R, Liu Y, Zhu T, Ma X, Liu R, Xu G, Meng L, Lu Y, Zhou J, Ma D (2008) TMTP1, a novel tumor-homing peptide specifically targeting metastasis. Clinical Cancer Research 14(17): 5494–5502. https://doi.org/10.1158/1078-0432.CCR-08-0233 [PubMed]
Zhao N, Qin Y, Liu H, Cheng Z (2018) Tumor-targeting peptides: Ligands for molecular imaging and therapy. Anti-Cancer Agents in Medicinal Chemistry 18(1): 74–86. https://doi.org/10.2174/1871520617666170419143459[PubMed]
Zhao R, Diop-Bove N, Visentin M, Goldman ID (2011) Mechanisms of membrane transport of folates into cells and across epithelia. Annual Review of Nutrition 31: 177–201. https://doi.org/10.1146/annurev-nutr-072610-145133[PubMed] [PMC]
 Русский
Русский
 English
English

