Улучшение противоопухолевой терапии за счет применения самонаводящегося петита PL3, проникающего в клетку
DOI:
https://doi.org/10.18413/rrpharmacology.9.10028Аннотация
Введение. Самонаводящиеся пептиды привлекли большое внимание для разработки неинвазивных и таргетных систем доставки лекарственных средств с целью минимизации системной токсичности лекарственных средств, предназначенных для лечения опухолей, и повышения их биодоступности. Это исследование направлено на улучшение противоопухолевого действия лекарственного средства, предназначенного для лечения рака предстательной железы, путем таргетной доставки лекарственного средства в системе носителе, состоящей из пептида PL3, проникающего в клетку путем самонаведения в опухоль.
Материалы и методы: Система доставки лекарственного средства была сконструирована с помощью твердофазного синтеза пептида, а затем охарактеризована с помощью масс-спектра и высокоэффективной жидкостной хроматографии. Была проведена оценка жизнеспособности клеток для оценки их цитотоксичности как в отношении опухолевых (клетки рака предстательной железы), так и в отношении нормальных клеток, а для исследования интернализации использовались конфокальный лазерный сканирующий микроскоп и проточный цитометр. Для проверки эффективности системы доставки лекарственного средства, содержащей лекарственное средство, был проведен биохимический анализ ингибирования фермента на целевом ферменте дигидрофолатредуктазе.
Результаты и их обсуждение. Полученные данные подтвердили успешный синтез и введение лекарственного средства в систему-носитель и продемонстрировали его высокую эффективность в отношении цитотоксического действия и ингибирования дигидрофолатредуктазы со значительным клеточным поглощением в клетках рака предстательной железы.
Заключение: лекарственное средство было доставлено к клеткам-мишеням рака предстательной железы с помощью PL3-функциональной системой доставки лекарственных средств, и позволило локализовать лекарственное средство в опухолевых клетках, не затрагивая нормальные клетки. Таким образом, результаты исследования подчеркнули важность систем доставки лекарственных средств в лечении опухолей.
Графическая аннотация
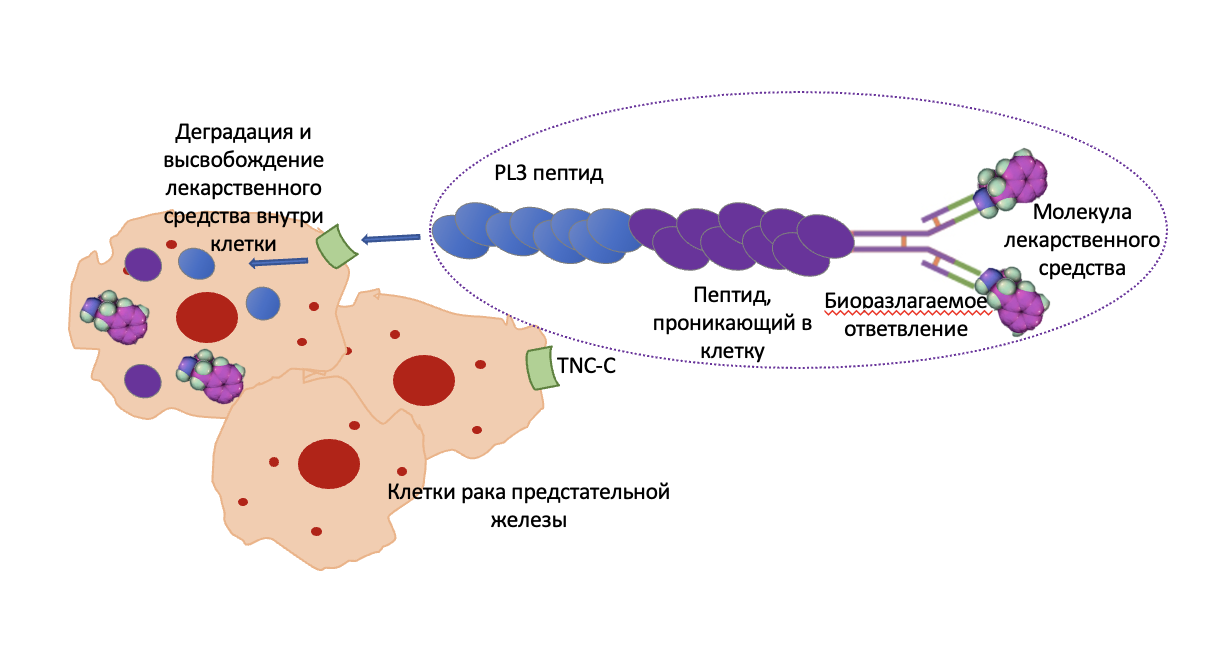
Ключевые слова:
химиотерапия рака, пептид, проникающий в клетку, система доставки лекарственных средств, рак предстательной железы, самонаправляющийся пептид в опухольБиблиографические ссылки
Al-azzawi S, Masheta D (2019) Designing a drug delivery system for improved tumor treatment and targeting by functionalization of a cell-penetrating peptide. Journal of Pharmaceutical Investigation 49: 643–654.
https://doi.org/10.1007/s40005-018-00424-w
Al-Azzawi S, Masheta D, Guildford A, Phillips G, Santin M (2019) Designing and characterization of a novel delivery system for improved cellular uptake by brain using dendronised Apo-E-derived peptide. Frontiers in Bioengineering and Biotechnology 7: 49. https://doi.org/10.3389/fbioe.2019.00049 [PubMed] [PMC]
Al-Azzawi S, Masheta D, Guildford A, Phillips G, Santin M (2020) A peptide-based nanocarrier for an enhanced delivery and targeting of flurbiprofen into the brain for the treatment of Alzheimer’s disease: An in vitro study. Nanomaterials 10(8): 1590. https://doi.org/10.3390/nano10081590 [PubMed] [PMC]
Al-Azzawi S, Masheta D, Guildford AL, Phillips G, Santin M (2018) Dendrimeric Poly(Epsilon-Lysine) Delivery systems for the enhanced permeability of flurbiprofen across the blood-brain barrier in Alzheimer’s disease. International Journal of Molecular Sciences 19(10): 3224. https://doi.org/10.3390/ijms19103224 [PubMed] [PMC]
Alqosaibi AI (2022) Nanocarriers for anticancer drugs: Challenges and perspectives. Saudi Journal of Biological Sciences 29(6): 103298. https://doi.org/10.1016/j.sjbs.2022.103298 [PubMed] [PMC]
Ayo A, Laakkonen P (2021) Peptide-based strategies for targeted tumor treatment and imaging. Pharmaceutics 13(4): 481. https://doi.org/10.3390/pharmaceutics13040481 [PubMed] [PMC]
Bolhassani A, Jafarzade BS, Mardani G (2017) In vitro and in vivo delivery of therapeutic proteins using cell penetrating peptides. Peptides, 87: 50–63. https://doi.org/10.1016/j.peptides.2016.11.011 [PubMed]
Dissanayake S, Denny WA, Gamage S, Sarojini V (2017) Recent developments in anticancer drug delivery using cell penetrating and tumor targeting peptides. Journal of Controlled Release 250: 62–76. https://doi.org/10.1016/j.jconrel.2017.02.006 [PubMed]
Dubikovskaya EA, Thorne SH, Pillow TH, Contag CH, Wender PA (2008) Overcoming multidrug resistance of small-molecule therapeutics through conjugation with releasable octaarginine transporters. Proceedings of the National Academy of Sciences 105(34): 12128–2133. https://doi.org/10.1073/pnas.0805374105 [PubMed] [PMC]
Dutta B, Barick KC, Hassan PA (2021) Recent advances in active targeting of nanomaterials for anticancer drug delivery. Advances in Colloid and Interface Science 296: 102509. https://doi.org/10.1016/j.cis.2021.102509 [PubMed]
Feng T, Zhao Ya (2017) Nanomaterial-based Drug Delivery Carriers for Cancer Therapy: Clinical Anticancer Drugs for Cancer Treatment, Springer, Singapore, pp. 7–13. https://doi.org/10.1007/978-981-10-3299-8
Gao Q, Feng J, Liu W, Wen C, Wu Y, Liao Q, Zou L, Sui X, Xie T, Zhang J, Hu Y (2022) Opportunities and challenges for co-delivery nanomedicines based on combination of phytochemicals with chemotherapeutic drugs in cancer treatment. Advanced Drug Delivery Reviews 188: 114445. https://doi.org/10.1016/j.addr.2022.114445[PubMed]
Garg NK, Singh B, Jain A, Nirbhavane P, Sharma R, Tyagi RK, Kushwah V, Jain S, Katare OP (2016) Fucose decorated solid-lipid nanocarriers mediate efficient delivery of methotrexate in breast cancer therapeuticsю. Colloids and Surfaces B: Biointerfaces 146: 114–126. https://doi.org/10.1016/j.colsurfb.2016.05.051 [PubMed]
Ghaz-Jahanian MA, Abbaspour-Aghdam F, Anarjan N, Berenjian A, Jafarizadeh-Malmiri H (2015) Application of chitosan-based nanocarriers in tumor-targeted drug delivery. Molecular Biotechnology 57(3): 201–218. https://doi.org/10.1007/s12033-014-9816-3 [PubMed]
Guidotti G, Brambilla L, Rossi D (2017) Cell-penetrating peptides: From basic research to clinics. Trends in Pharmacological Sciences 38(4): 406–424. https://doi.org/10.1016/j.tips.2017.01.003 [PubMed]
Hannoodee M, Meenal M (2022) Methotrexate. in, StatPearls (StatPearls Publishing) [PMC]
Kamalidehghan B, Ghafouri-Fard S, Motevaseli E, Ahmadipour F (2018) Inhibition of human prostate cancer (PC-3) cells and targeting of PC-3-derived prostate cancer stem cells with koenimbin, a natural dietary compound from Murraya koenigii (L) Spreng. Drug Design, Development and Therapy 12: 1119–1133. https://doi.org/10.2147/DDDT.S156826 [PubMed] [PMC]
Kebebe D, Liu Y, Wu Y, Vilakhamxay M, Liu Z, Li J (2018) Tumor-targeting delivery of herb-based drugs with cell-penetrating/tumor-targeting peptide-modified nanocarriers. International Journal of Nanomedicine, 13: 1425–1442. https://doi.org/10.2147/IJN.S156616 [PubMed] [PMC]
Kondo E, Iioka H, Saito K (2021) Tumor‐homing peptide and its utility for advanced cancer medicine. Cancer Science 112(6): 2118–2125. https://doi.org/10.1111/cas.14909 [PubMed] [PMC]
Le Joncour V, Laakkonen P (2018) Seek & destroy, use of targeting peptides for cancer detection and drug delivery. Bioorganic & Medicinal Chemistry 26(10): 2797–2806. https://doi.org/10.1016/j.bmc.2017.08.052 [PubMed]
Lee HL, Dubikovskaya EA, Hwang H, Semyonov AN, Wang H, Jones LR, Twieg RJ, Moerner WE, Wender PA (2008) Single-molecule motions of oligoarginine transporter conjugates on the plasma membrane of Chinese hamster ovary cells. Journal of the American Chemical Society 130(29): 9364–9370. https://doi.org/10.1021/ja710798b [PubMed] [PMC]
Lian B, Wei H, Pan R, Sun J, Zhang B, Wu J, Li X, Tian G (2021) Galactose modified liposomes for effective co-delivery of doxorubicin and combretastatin A4. International Journal of Nanomedicine 16: 457–467. https://doi.org/10.2147/IJN.S283793 [PubMed] [PMC]
Lindgren M, Rosenthal-Aizman K, Saar K, Eiríksdóttir E, Jiang Y, Sassian M, Ostlund P, Hällbrink M, Langel U (2006) Overcoming methotrexate resistance in breast cancer tumour cells by the use of a new cell-penetrating peptide. Biochemical Pharmacology 71(4): 416-425. https://doi.org/10.1016/j.bcp.2005.10.048 [PubMed]
Lingasamy P, Tobi A, Kurm K, Kopanchuk S, Sudakov A, Salumäe M, Rätsep T, Asser T, Bjerkvig R, Teesalu T (2020) Tumor-penetrating peptide for systemic targeting of Tenascin-C. Scientific Reports 10: 1–13. https://doi.org/10.1038/s41598-020-62760-y [PubMed] [PMC]
Litwin MS, Tan HJ (2017) The diagnosis and treatment of prostate cancer: A review. JAMA 317(24): 2532–2542. https://doi.org/10.1001/jama.2017.7248 [PubMed]
Madamsetty VS, Mukherjee A, Mukherjee S (2019) Recent trends of the bio-inspired nanoparticles in cancer theranostics. Frontiers in Pharmacology 10: 1264. https://doi.org/10.3389/fphar.2019.01264 [PubMed] [PMC]
Meikle ST, Perugini V, Guildford AL, Santin M (2011) Synthesis, characterisation and in vitro anti-angiogenic potential of dendron VEGF blockers. Macromolecular Bioscience 11(12): 1761–1765. https://doi.org/10.1002/mabi.201100267 [PubMed]
Merrifield RB (1965) Automated synthesis of peptides. Science 150(3693): 178–185. https://doi.org/10.1126/science.150.3693.178 [PubMed]
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods 65(1-2): 55–63. https://doi.org/10.1016/0022-1759(83)90303-4 [PubMed]
Ramsey JD, Flynn NH (2015) Cell-penetrating peptides transport therapeutics into cells. Pharmacology & Therapeutics 154: 78–86. https://doi.org/10.1016/j.pharmthera.2015.07.003 [PubMed]
Regberg J, Srimanee A, Langel U (2012) Applications of cell-penetrating peptides for tumor targeting and future cancer therapies. Pharmaceuticals (Basel) 5(9): 991–1007. https://doi.org/10.3390/ph5090991 [PubMed]
Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B (2003) Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. The Journal of Biological Chemistry 278(1): 585–590. https://doi.org/10.1074/jbc.M209548200 [PubMed]
Rodríguez H, Suarez M, Albericio F (2010) A convenient microwave-enhanced solid-phase synthesis of short chain N-methyl-rich peptides. Journal of Peptide Science 16(3): 136–140. https://doi.org/10.1002/psc.1209 [PubMed]
Shin MC, Zhang J, Min KA, Lee K, Byun Y, David AE, He H, Yang VC (2014) Cell-penetrating peptides: achievements and challenges in application for cancer treatment. Journal of Biomedical Materials Research. Part A 102(2): 575–587. https://doi.org/10.1002/jbm.a.34859 [PubMed] [PMC]
Silverman R, Holladay M (2014) The organic chemistry of drug design and drug action, Elsevier Inc., USA 517 pp. https://doi/10.1016/C2009-0-64537-2
Soler M, González-Bártulos M, Figueras E, Ribas X, Costas M, Massaguer A, Planas M, Feliu L (2015) Enzyme-triggered delivery of chlorambucil from conjugates based on the cell-penetrating peptide BP16. Organic & Biomolecular Chemistry 13(5): 1470–1480. https://doi.org/10.1039/c4ob01875c [PubMed]
Stanzl EG, Trantow BM, Vargas JR, Wender PA (2013) Fifteen years of cell-penetrating, guanidinium-rich molecular transporters: basic science, research tools, and clinical applications. Accounts of Chemical Research 46(12): 2944–2954. https://doi.org/10.1021/ar4000554 [PubMed] [PMC]
Tang L, Li J, Zhao Q, Pan T, Zhong H, Wang W (2021) Advanced and innovative nano-systems for anticancer targeted drug delivery. Pharmaceutics 13(8): 1151. [PubMed] [PMC]
Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E (2009) C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proceedings of the National Academy of Sciences of the United States of America 106(38): 16157–16162. https://doi.org/10.1073/pnas.0908201106 [PubMed] [PMC]
Torchilin VP, Levchenko TS, Rammohan R, Volodina N, Papahadjopoulos-Sternberg B, D'Souza GG (2003) Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proceedings of the National Academy of Sciences of the United States of America 100(4): 1972–1977. https://doi.org/10.1073/pnas.0435906100[PubMed] [PMC]
Vargas JR, Stanzl EG, Teng NN, Wender PA (2014) Cell-penetrating, guanidinium-rich molecular transporters for overcoming efflux-mediated multidrug resistance. Molecular Pharmaceutics 11(8): 2553–2565.https://doi.org/10.1021/mp500161z [PubMed] [PMC]
Wada A, Terashima T, Kageyama S, Yoshida T, Narita M, Kawauchi A, Kojima H (2019) Efficient prostate cancer therapy with tissue-specific homing peptides identified by advanced phage display technology. Molecular Therapy Oncolytics 12: 138–146. https://doi.org/10.1016/j.omto.2019.01.001 [PubMed] [PMC]
Wender PA, Cooley CB, Geihe EI (2012) Beyond cell penetrating peptides: designed molecular transporters. Drug Discovery Today. Technologies 9(1): e49–e55. https://doi.org/10.1016/j.ddtec.2011.07.004 [PubMed] [PMC]
Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB (2000) The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proceedings of the National Academy of Sciences of the United States of America 97(24): 13003–13008. https://doi.org/10.1073/pnas.97.24.13003 [PubMed] [PMC]
Xu L, Zhang H, Wu Y (2014) Dendrimer advances for the central nervous system delivery of therapeutics. ACS Chemical Neuroscience 5(1): 2–13. https://doi.org/10.1021/cn400182z [PubMed] [PMC]
Yang W, Luo D, Wang S, Wang R, Chen R, Liu Y, Zhu T, Ma X, Liu R, Xu G, Meng L, Lu Y, Zhou J, Ma D (2008) TMTP1, a novel tumor-homing peptide specifically targeting metastasis. Clinical Cancer Research 14(17): 5494–5502. https://doi.org/10.1158/1078-0432.CCR-08-0233 [PubMed]
Zhao N, Qin Y, Liu H, Cheng Z (2018) Tumor-targeting peptides: Ligands for molecular imaging and therapy. Anti-Cancer Agents in Medicinal Chemistry 18(1): 74–86. https://doi.org/10.2174/1871520617666170419143459[PubMed]
Zhao R, Diop-Bove N, Visentin M, Goldman ID (2011) Mechanisms of membrane transport of folates into cells and across epithelia. Annual Review of Nutrition 31: 177–201. https://doi.org/10.1146/annurev-nutr-072610-145133[PubMed] [PMC]
 Русский
Русский
 English
English

