Pancreatic β-cell protective effect of novel GABA derivatives in rats with type 2 diabetes
DOI:
https://doi.org/10.18413/rrpharmacology.9.10042Abstract
Introduction: Gamma-aminobutyric acid (GABA) and GABAergic compounds emerged as potential therapeutic agents for diabetes mellitus and its complications. GABA acts as an inhibitory neurotransmitter in the central nervous system and as an extracellular signaling molecule in pancreatic islets, exerting beneficial effects on insulin secretion, glucagon production, apoptosis, beta-cell survival, and regeneration.
Aim: This study aimed to compare the efficacy of GABA and GABAergic compounds as pancreatic β-cell protective agents in aged rats (18 months) with prolonged hyperglycemia induced by streptozotocin-nicotinamide injection.
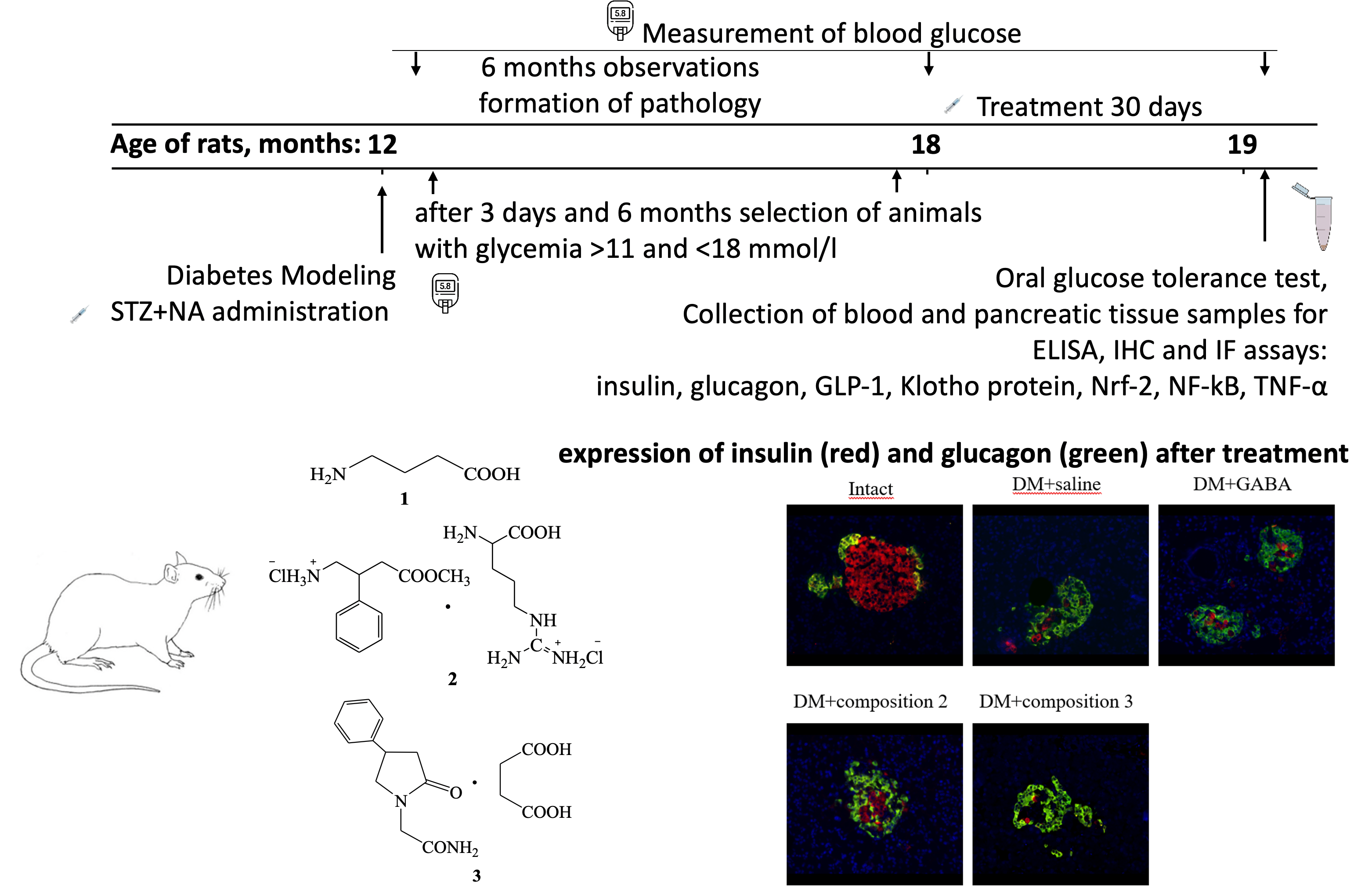
Materials and Methods: Male outbred albino rats aged 12 months were intraperitoneally injected with streptozotocin (65 mg/kg) and nicotinamide (230 mg/kg). Over the next 6 months, the level of glycemia was monitored every 4 weeks. Further, rats with postprandial glycemia levels between 11 and 18 mmol/L were selected. The experimental groups were treated with GABA and GABAergic compounds (compositions 2 and 3) for 1 month, while the control group received saline. An oral glucose tolerance test (OGTT) was performed after treatment. Blood and samples of the pancreatic tissue (splenic part) were collected for enzyme-linked immunosorbent assay (GLP-1, TNF-α serum level and NF-κB, Nrf2, Klotho tissue homogenate level), immunohistochemistry (islet NF-κB, Nrf2 and Klotho protein expression) and immunofluorescence assays (islet insulin and glucagon expression).
Results: The research findings demonstrate significant hypoglycemic effects of the studied GABA derivatives in aged rats with prolonged hyperglycemia. These GABA derivatives effects were accompanied with increased GLP-1 production and improved pancreatic β-cell function and mass. Furthermore, elevated levels of Klotho protein and Nrf2 transcription factor, along with the suppression of NF-κB transcription factor after treatment, may play a crucial role in the β-cellprotective effects of these GABA derivatives.
Conclusion: Novel GABA derivatives exhibit significant pancreatic β-cell protective effects that may be mediated by enhanced GLP-1, Klotho protein, and Nrf2 transcription factor, and suppressed NF-κB transcription factor. These results highlight the potential of GABA derivatives as promising therapeutic agents for managing diabetes mellitus and its associated complications.
Graphical Abstract
Keywords:
diabetes mellitus, GLP-1, insulin, Klotho protein, NF-κB, Nrf2, streptozotocinReferences
Abraham CR, Li A (2022) Aging-suppressor Klotho: Prospects in diagnostics and therapeutics. Ageing Research Reviews 82: 101766. https://doi.org/10.1016/j.arr.2022.101766 [PubMed]
Adoga JO, Channa ML, Nadar A (2022) Type-2 diabetic rat heart: The effect of kolaviron on mTOR-1, P70S60K, PKC-α, NF-kB, SOD-2, NRF-2, eNOS, AKT-1, ACE, and P38 MAPK gene expression profile. Biomedicine & Pharmacotherapy 148: 112736. https://doi.org/10.1016/j.biopha.2022.112736 [PubMed]
Al-Kuraishy HM, Hussian NR, Al-Naimi MS, Al-Gareeb AI, Al-Mamorri F, Al-Buhadily AK (2021) The potential role of pancreatic γ-aminobutyric acid (GABA) in diabetes mellitus: A critical reappraisal. International Journal of Preventive Medicine 12: 19. https://doi.org/10.4103/ijpvm.IJPVM_278_19 [PubMed] [PMC]
Antoni FA (2022) The case for clinical trials with novel GABAergic drugs in diabetes mellitus and obesity. Life (Basel, Switzerland) 12(2): 322. https://doi.org/10.3390/life12020322 [PubMed] [PMC]
Arrojo e Drigo R (2021) Probing β-Cell biology in space and time. Diabetes 70(10): 2163–2173. https://doi.org/10.2337/dbi21-0008 [PubMed] [PMC]
Dai C, Hang Y, Shostak A, Poffenberger G, Hart N, Prasad N, Phillips N, Levy SE, Greiner DL, Shultz LD, Bottino R, Kim SK, Powers AC (2017) Age-dependent human β cell proliferation induced by glucagon-like peptide 1 and calcineurin signaling. The Journal of Clinical Investigation 127(10): 3835–3844. https://doi.org/10.1172/JCI91761[PubMed] [PMC]
Dedov II, Shestakova MV, Vikulova OK, Zheleznyakova AV, Isakov MA, Sazonova DV, Mokrysheva NG (2023) Diabetes mellitus in the Russian Federation: Dynamics of epidemiological indicators according to the federal register of diabetes mellitus for the period 2010-2022. Diabetes Mellitus [Sakharnyj Diabet] 26(2): 104–123. https://doi.org/10.14341/dm13035 [in Russian]
Donate-Correa J, Martín-Carro B, Cannata-Andía JB, Mora-Fernández C, Navarro-González JF (2023) Klotho, oxidative stress, and mitochondrial damage in kidney disease. Antioxidants (Basel, Switzerland) 12(2): 239. https://doi.org/10.3390/antiox12020239 [PubMed] [PMC]
Gao W, Guo L, Yang Y, Wang Y, Xia S, Gong H, Zhang BK, Yan M (2022) Dissecting the crosstalk between Nrf2 and NF-κB response pathways in drug-induced toxicity. Frontiers in Cell and Developmental Biology 9: 809952. https://doi.org/10.3389/fcell.2021.809952 [PubMed] [PMC]
Indrowati M, Pratiwi R, Rumiyati, Astuti P (2017) Levels of blood glucose and insulin expression of beta-cells in streptozotocin-induced diabetic rats treated with ethanolic extract of artocarpus altilis leaves and GABA. Pakistan Journal of Biological Sciences: PJBS 20(1): 28–35. https://doi.org/10.3923/pjbs.2017.28.35 [PubMed]
Liu W, Son DO, Lau HK, Zhou Y, Prud'homme GJ, Jin T, Wang Q (2017) Combined oral administration of GABA and DPP-4 inhibitor prevents beta cell damage and promotes beta cell regeneration in mice. Frontiers in Pharmacology 8: 362. https://doi.org/10.3389/fphar.2017.00362 [PubMed] [PMC]
Maltese G, Psefteli PM, Rizzo B, Srivastava S, Gnudi L, Mann GE, Siow RC (2017) The anti-ageing hormone klotho induces Nrf2-mediated antioxidant defences in human aortic smooth muscle cells. Journal of Cellular and Molecular Medicine 21(3): 621–627. https://doi.org/10.1111/jcmm.12996 [PubMed] [PMC]
Prud'homme GJ, Glinka Y, Kurt M, Liu W, Wang Q (2017) The anti-aging protein Klotho is induced by GABA therapy and exerts protective and stimulatory effects on pancreatic beta cells. Biochemical and Biophysical Research Communications 493(4): 1542–1547. https://doi.org/10.1016/j.bbrc.2017.10.029 [PubMed]
Prud’homme GJ, Glinka Y, Udovyk O, Hasilo C, Paraskevas S, Wang Q (2014) GABA protects pancreatic beta cells against apoptosis by increasing SIRT1 expression and activity. Biochemical and Biophysical Research Communications 452(3): 649–654. https://doi.org/10.1016/j.bbrc.2014.08.135 [PubMed]
Prud'homme GJ, Kurt M, Wang Q (2022) Pathobiology of the klotho antiaging protein and therapeutic considerations. Frontiers in Aging 3: 931331. https://doi.org/10.3389/fragi.2022.931331 [PubMed] [PMC]
Robertson RP (2023) Nrf2 and antioxidant response in animal models of type 2 diabetes. International Journal of Molecular Sciences 24(4): 3082. https://doi.org/10.3390/ijms24043082 [PubMed] [PMC]
Soltani N, Qiu H, Aleksic M, Glinka Y, Zhao F, Liu R, Li Y, Zhang N, Chakrabarti R, Ng T, Jin T, Zhang H, Lu WY, Feng ZP, Prud’homme GJ, Wang Q (2011) GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proceedings of the National Academy of Sciences of the United States of America 108(28): 11692–11697. https://doi.org/10.1073/pnas.1102715108 [PubMed] [PMC]
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ (2022) IDF diabetes atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Research and Clinical Practice 183: 109119. https://doi.org/10.1016/j.diabres.2021.109119 [PubMed]
Typiak M, Piwkowska A (2021) Antiinflammatory actions of klotho: Implications for therapy of diabetic nephropathy. International Journal of Molecular Sciences 22(2): 956. https://doi.org/10.3390/ijms22020956 [PubMed] [PMC]
van der Horst D, Carter-Timofte ME, van Grevenynghe J, Laguette N, Dinkova-Kostova AT, Olagnier D (2022) Regulation of innate immunity by Nrf2. Current Opinion in Immunology 78: 102247. https://doi.org/10.1016/j.coi.2022.102247 [PubMed]
Zubkiewicz-Kucharska A, Wikiera B, Noczyńska A (2021) Soluble klotho is decreased in children with type 1 diabetes and correlated with metabolic control. Frontiers in endocrinology 12: 709564. https://doi.org/10.3389/fendo.2021.709564[PubMed] [PMC]
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Иван Н. Тюренков, Дмитрий А. Бакулин, Юлия И. Великородная, Александр В. Борисов, Елизавета А. Абросимова, Алексей В. Смирнов, Григорий Л. Снигур, Святослав С. Сурин, Дарья А. Кавалерова, Ольга С. Васильева

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

