Modeling experimental glaucoma for screening studies of antiglaucomatous activity
DOI:
https://doi.org/10.18413/rrpharmacology.9.10043Abstract
Introduction: In vivo screening studies, in which the efficacy of dozens of drugs is tested to select several applicants for further study of their safety in humans, are the main stage in the study of the pharmacodynamics of promising antiglaucoma drugs. This imposes a number of specific requirements both on experimental models of glaucoma and on laboratory animals used in the experiment.
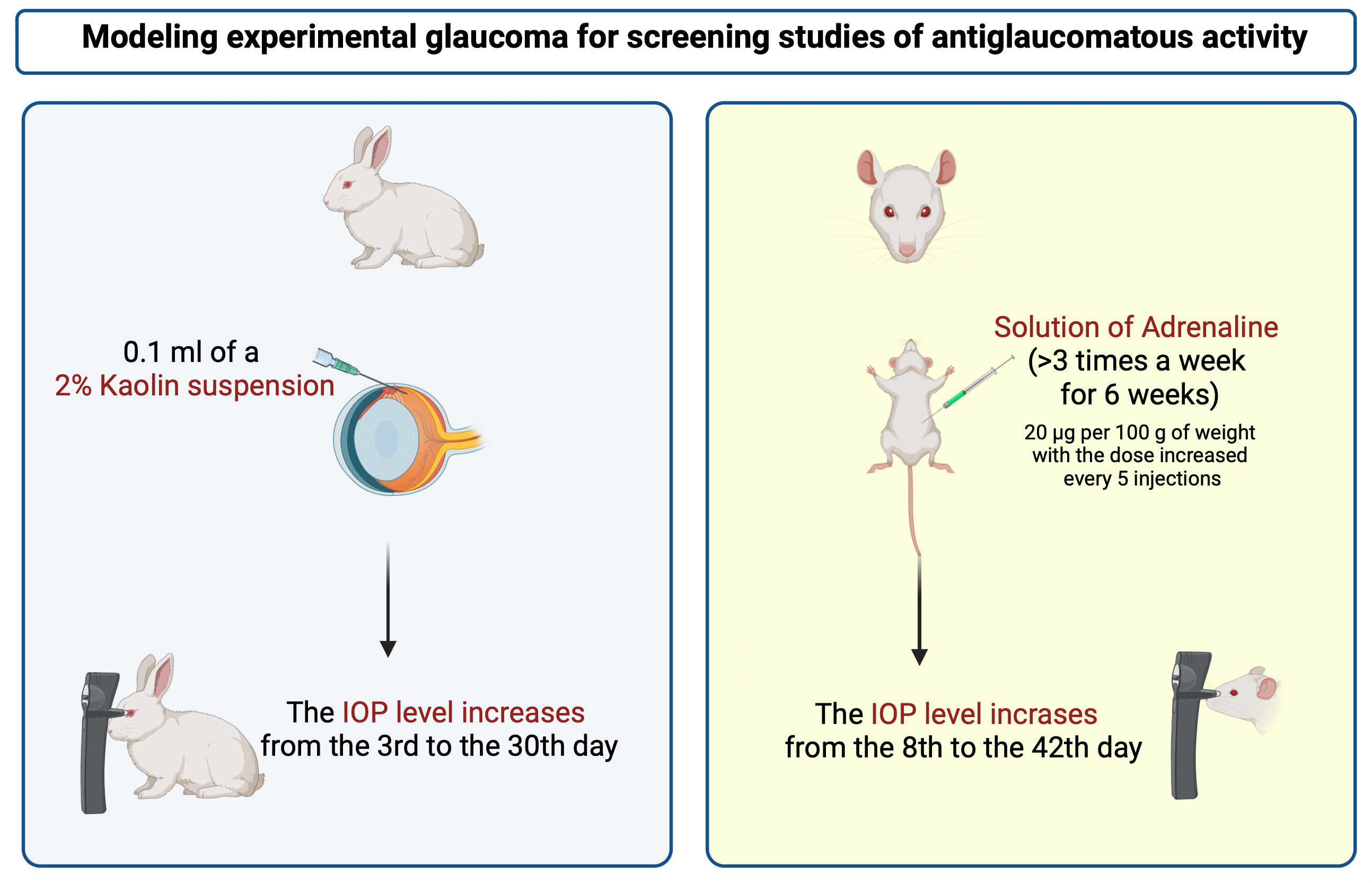
Materials and Methods: 32 male rabbits of the Soviet Сhinchilla breed, 6 male albino rabbits weighing 3-3.5 kg, and 20 outbred white rats weighing 220-250 g were used in total in experiments to reproduce the glaucoma process. All manipulations on the rabbit eye were performed by an ophthalmologist under general anesthesia with telazol. Triamcinolone (vitreous injection) was used to simulate glaucoma in rabbits, lauromacrogol 400 or fine kaolin (anterior chamber injection) was used to simulate glaucoma in rabbits; adrenaline hydrochloride (intraperitoneal administration) was used to simulate glaucoma in rats.
Results and Discussion: Double intravitreal administration of a suspension of triamcinolone at a dose of 4 mg was the most attractive model in terms of the technique of reproducing the pathology and the results obtained in modeling glaucoma in rabbits. However, this model did not produce a stable increase in intraocular pressure (IOP). Doubling the dose of triamcinolone and replacing chinchilla rabbits with albinos did not lead to a positive result. The introduction of the venous sclerosing drug lauromacrogol 400 into the anterior chamber of the eye proved to be ineffective either. The introduction of finely dispersed kaolin into the anterior chamber of the eye of rabbits led to a persistent increase in IOP. The intraperitoneal administration of epinephrine hydrochloride to rats according to the described method gave no stable results. The increase in IOP became stable only after a significant increase in the dose of adrenaline.
Conclusion: The conducted studies of four models of glaucoma and their three modifications in animals made it possible to select two of them, which contributed to a stable and fairly long-term increase in IOP in rabbits (introduction of finely dispersed kaolin into the anterior chamber of the eye) and rats (adrenaline-induced model).
Graphical Abstract
Keywords:
glaucoma, intraocular pressure, experimental model of glaucomaReferences
Al-Rajhi A, Ambrus A, Daly M, Lum FC (2020) Primary open-angle glaucoma preferred practice pattern. American Academy of Ophthalmology, USA, pp. 71–150.
Alyabyeva ZhYu, Romanova TB, Lipatova VA, Botchei VM (2015) Experimental models of glaucoma in the research of a new neuroprotection treatment. Russian Journal of Clinical Ophthalmology [Rossiiskii Zhurnal Klinicheskoi Oftal'mologhii] 15(3): 145–149. [in Russian]
Aznabaev BM, Aznabaev MT, Krieger GS, Solomatnikova SR (1998) Method for creating a model of experimental glaucoma. Russian patent No. 2164396, 4 pp. [in Russian]
Bouhenni R, Dunmire J, Sewell A, Edward DP (2012) Animal models of glaucoma. Journal of Biomedicine and Biotechnology 2012: 692609. https://doi.org/10.1155/2012/692609 [PubMed] [PMC]
Daimofl T, Kazama M, Miyajima Y, Nakaflo M (1997) Immunocytochemical localization of thrombomodulin in the aqueous humor passage of the rat eye. Histochemistry and Cell Biology 108(2): 121–131. https://doi.org/10.1007/s004180050153 [PubMed]
Gazizova IR, Alekseyev VN, Nikitin DN (2013) Experimental reproduction of the glaucomatous process. Ophthalmology Reports [Oftal'mologicheskie Vedomosti] 6(3): 43–50. [in Russian]
Izzotti A, Sacca SC, Longobardi M, Cartiglia C (2010) Mitochondrial damage in the trabecular meshwork ofpatients with glaucoma. Archives of Ophthalmology 128(6): 724–730. https://doi.org/10.1001/archophthalmol.2010.87 [PubMed]
Jones R,Rhee DJ (2006) Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Current Opinion in Ophthalmology 17(2): 163–167. https://doi.org/10.1097/01.icu.0000193079.55240.18 [PubMed]
Kersey JP, Broadway DC (2006) Corticosteroid-induced glaucoma: a review of the literature. Eye 20(4): 407–416. https://doi.org/10.1038/sj.eye.6701895 [PubMed]
Kurysheva NI (2020) Carbonic anhydrase inhibitors in the treatment of glaucoma. Review. Part I. Ophthalmology in Russia [Oftal'mologiya] 17(S3): 542–549. https://doi.org/10.18008/1816-5095-2020-3S-542-549 [in Russian]
Livne-Bar I, Guo X, Sivak JM (2012) Establishing a new mouse glaucoma model. Investigative Ophtalmology and Visual Science 53(14): 2484.
Mikheytseva IN (2014) Glaucoma modeling and adrenal stress. Journal of Clinical Medicine Research 4: 427–437.
Morrison JC, Fraunfelder FW, Milne ST, Moore CG (1995) Limbal microvasculature of the rat eye. Investigative Ophthalmology and Visual Science 2: 751–756. [PubMed]
Petrov SYu, Subbot AM, Gabashvili AN, Volzhanin AV, Vitkov AA (2017) Rat models of glaucomatous optic neuropathy. National Journal Glaucoma 16(4): 79–85. https://doi.org/10.53432/2078-4104-2023-22-1-115-128
Prigogina AL (1966) Pathological anatomy and pathogenesis of glaucoma. Medicine, Moscow, p. 173–174. [in Russian]
Song Z, Gao H, Liu H, Sun X (2011) Metabolomics of rabbit aqueous humor after administration of glucocorticosteroid. Current Eye Research 36(6): 563–570. https://doi.org/10.3109/02713683.2011.566410[PubMed]
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 121(11): 2081–2090. https://10.1016/j.ophtha.2014.05.013 [PubMed]
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Vladimir N. Fedorov, Mikhail K. Korsakov, Vladimir P. Vdovichenko, Salavat S. Suleimanov, Alena N. Tyushina, Anastasiya A. Popova

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

