Antiparkinsonian activity of selective MAO-B inhibitors of different chemical structures.
DOI:
https://doi.org/10.18413/rrpharmacology.10.540Abstract
Introduction: Parkinson’s disease (PD) is a chronic neurodegenerative disease of the central nervous system, the pathogenesis of which is associated with the death of dopaminergic neurons of the midbrain substantia nigra. The mainstay of therapy for PD is levodopa. However, in the initial stages of PD or in case of levodopa intolerance, MAO B inhibitors are used. Purpose of the study was to determine antiparkinsonian activity of newly synthesized selective MAO-B inhibitors in vivo on the model of experimental parkinsonism in white mice.
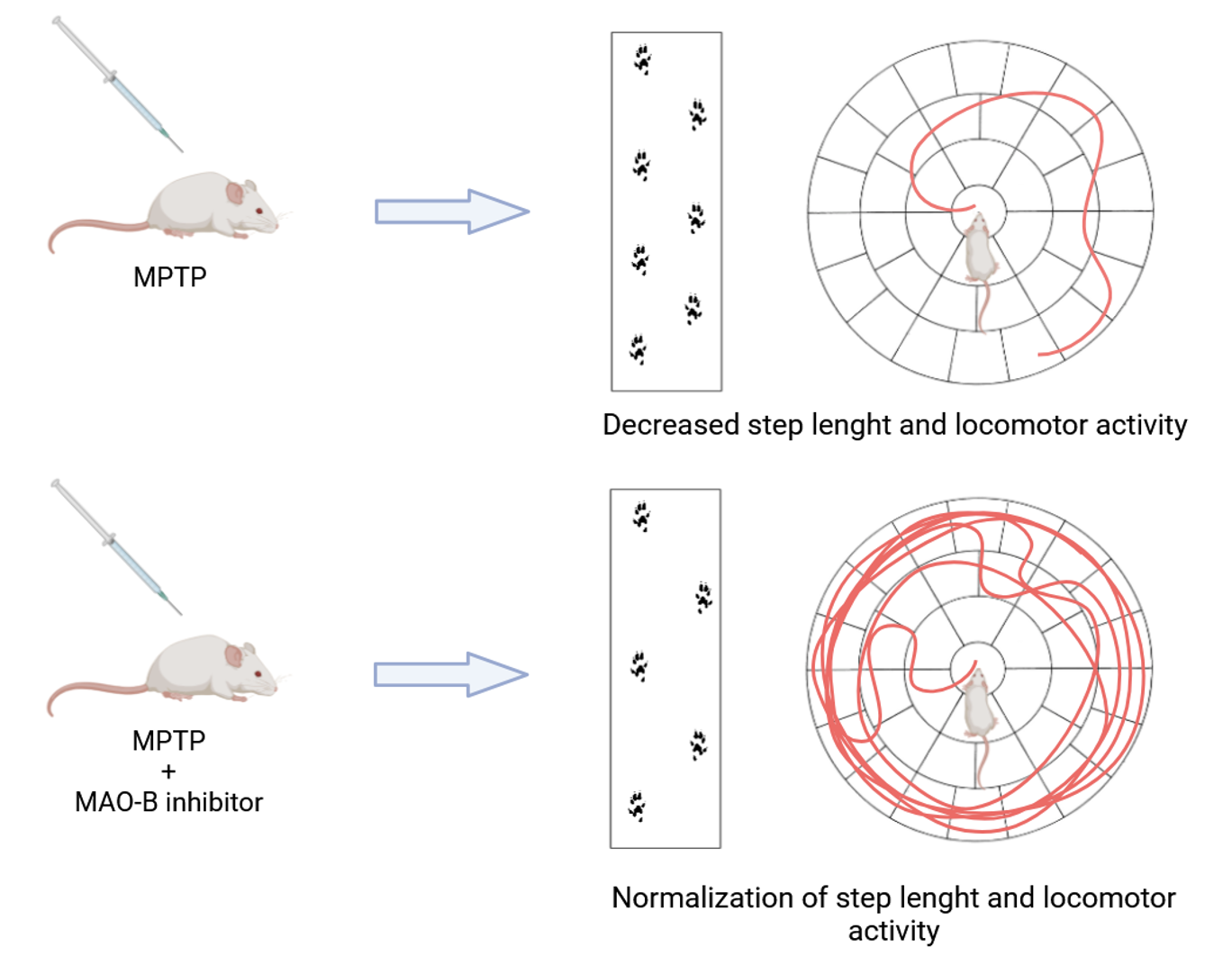
Materials and Methods: Parkinsonian syndrome in mice was modeled by systemic administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). As the criteria for its evaluation, we used the determination of muscle rigidity severity by step length of mice and evaluation of oligokinesia severity and emotional and mental disturbances in the open field test. A total of 9 candidate compounds were studied, assigned with laboratory codes S1-S5, S9, S10, S14, and S15. Rasagiline was used as a comparison drug.
Results and Discussion: Of the 9 compounds used at a dosage of 2mg/kg, the degree of rigidity was significantly reduced by compounds S1, S9, and S15; locomotor activity was restored to the level reached through rasagiline only by compound S9; the decline in exploratory activity was prevented only by S9 and to some extent by S5.
Conclusion: Only compound S9 having benzenesulfonamide chemotype showed significant therapeutic potential in a model of experimental parkinsonism and only in relation to it additional studies can be planned.
Graphical Abstract
Keywords:
Parkinson’s disease, MAO-B inhibitors, experimental parkinsonism, benzenesulfonamide derivativeReferences
Armstrong MJ, Okun MS (2020) Diagnosis and treatment of Parkinson disease: A review. JAMA 323(6): 548–560. https://doi.org/10.1001/jama.2019.22360 [PubMed]
Chew ZX, Lim CL, Ng KY, Chye SM, Ling APK, Koh RY (2023) The role of monoamine oxidase b inhibitors in the treatment of Parkinson’s disease - an update. CNS & Neurological Disorders Drug Targets 22(3): 329–352. https://doi.org/10.2174/1871527321666211231100255 [PubMed]
Glantz S (1998) Medical and Biological Statistics, Moscow, Practica, 459pp. [in Russian]
Gómez-Benito M, Granado N, García-Sanz P, Michel A, Dumoulin M, Moratalla R (2020) Modeling Parkinson’s disease with the alpha-synuclein protein. Frontiers in Pharmacology 11: 356. https://doi.org/10.3389/fphar.2020.00356 [PubMed] [PMC]
Jost WH (2022) A critical appraisal of MAO-B inhibitors in the treatment of Parkinson’s disease. Journal of Neural Transmission (Vienna, Austria: 1996) 129(5–6): 723–736. https://doi.org/10.1007/s00702-022-02465-w[PubMed] [PMC]
Mironov AN (2012) Guide on conducting preclinical trial of medications. Part 1. Ministry of Health and Social Development of the Russian Federation, Nauchny Centr Expertizy Sredstv Medicinskogo Primeneniya [Scientific Center for Expert Evaluation of Medicinal Products], Polygraph Plus, Moscow, 944 pp.
Ramesh S, Arachchige ASPM (2023) Depletion of dopamine in Parkinson’s disease and relevant therapeutic options: A review of the literature. AIMS Neuroscience 10(3): 200–231. https://doi.org/10.3934/Neuroscience.2023017 [PubMed] [PMC]
Vanderah TW (2024) Katzung’s Basic & Clinical Pharmacology, 16th Edition, Mc Graw Hill, 517-537
Yan R, Cai H, Cui Y, Su D, Cai G, Lin F, Feng T (2023) Comparative efficacy and safety of monoamine oxidase type B inhibitors plus channel blockers and monoamine oxidase type B inhibitors as adjuvant therapy to levodopa in the treatment of Parkinson’s disease: a network meta-analysis of randomized controlled trials. European Journal of Neurology 30(4): 1118–1134. https://doi.org/10.1111/ene.15651 [PubMed]
Youdim MBH, Riederer PF (2007) Monoamine oxidase A and B inhibitors in Parkinson’s disease. In: Koller WC, Melamed E (eds), Handbook of Clinical Neurology. Parkinson’s Disease and Related Disorders, Part II. Elsevier, 93–120. https://doi.org/10.1016/S0072-9752(07)84034-6
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Fedorov VN, Korsakov MK, Shetnev AA, Petukhov SS, Volkhin NN, Vdovichenko VP, Khokhlova AA, Suleymanov SS

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

