The Identification and synthesis of metabolites of 4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonamide
DOI:
https://doi.org/10.18413/rrpharmacology.10.498Abstract
Introduction: 4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonamide is a new selective type II carbonic anhydrase inhibitor with local action through instillation into the eyes. For a complete pharmacokinetic study of this drug, it is necessary to detect and synthesize its metabolites for their systemic exposure evaluation.
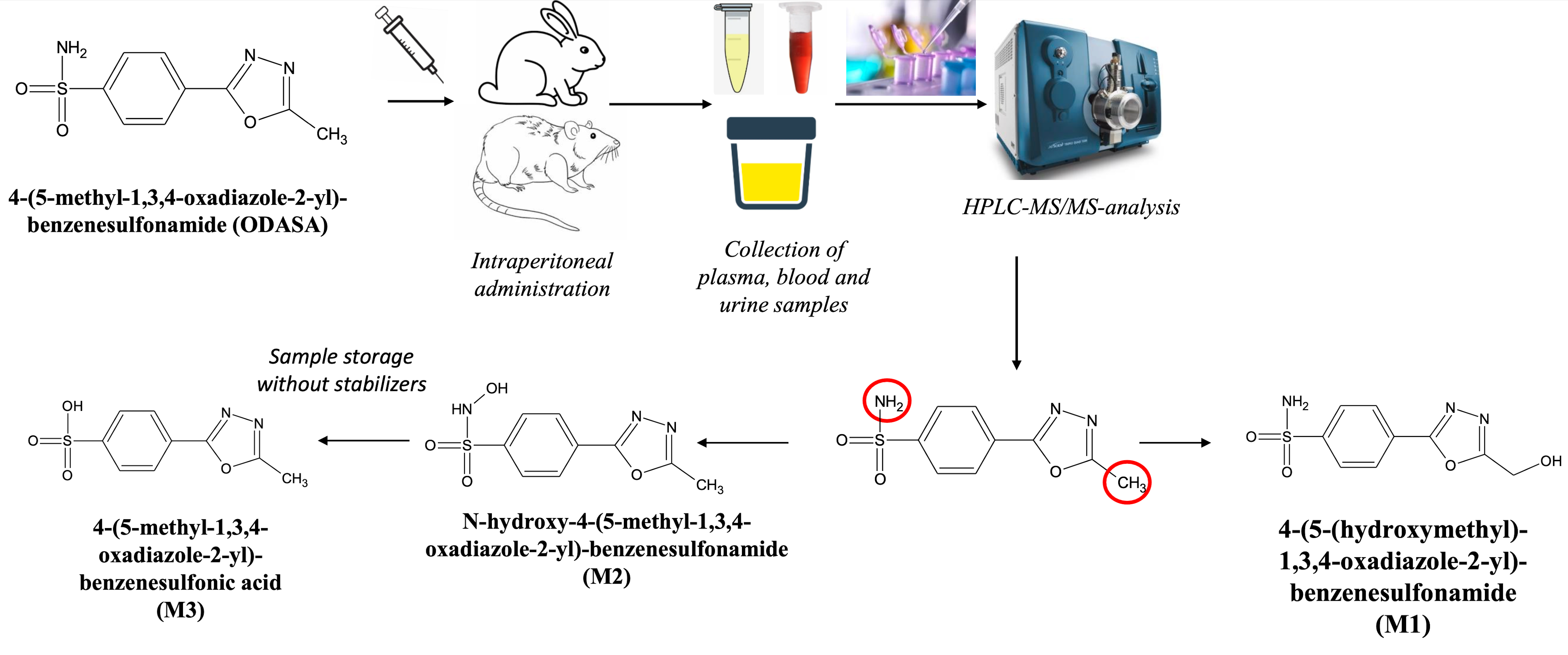
Materials and Methods: The investigation was performed on 6 Wistar rats and 6 Soviet Chinchilla rabbits. The drug in the form of a 1% suspension was administered by intraperitoneal injection. Blood was sampled in a volume of 0.2 mL at the following time points: before administration and 1 h, 2 h, 4 h, 24 h after administration. Then 150 µL of each sample was centrifuged to produce plasma. Urine was simultaneously sampled in rats using metabolic cells: before administration and at intervals of 0-2 h, 2-4 h, 4-6 h, 6-24 h after administration of the drug. The identification of metabolites in these objects was performed using HPLC-MS/MS. Then the detected biotransformation products were synthesized. The structure of the obtained substances was confirmed by NMR spectroscopy and high-resolution mass spectrometry. At the final stage, animal biological fluids and model samples with the addition of the synthesized compounds were analyzed using HPLC-MS/MS to establish the structure of metabolites.Results and Discussion: N-hydroxy-4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonamide, 4-(5-(hydroxymethyl)-1,3,4-oxadiazole-2-yl)-benzenesulfonamide and 4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonic acid were identified and synthesized. The complete coincidence of the structure of metabolites and the synthesized substances was established as a result of their comparison in retention time, the ratio of the areas of chromatographic peaks at the main MRM transitions, as well as mass spectra.Conclusion: N-hydroxy-4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonamide and 4-(5-(hydroxymethyl)-1,3,4-oxadiazole-2-yl)-benzenesulfonamide are products of biotransformation of the studied drug. It was found that 4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonic acid is formed by decomposition of N-hydroxymetabolite in urine samples during the collection process.
Graphical Abstract
Keywords:
biotransformation, selective carbonic anhydrase II inhibitor, HPLC-MS/MS, N-hydroxysulfonamideReferences
Alsibaee AM, Aljohar HI, Attwa MW, Abdelhameed AS, Kadi AA (2023) Investigation of fenebrutinib metabolism and bioactivation using MS3 methodology in ion trap LC/MS. Molecules 28(10): 4225. https://doi.org/10.3390/molecules28104225 [PubMed] [PMC]
Begou O, Drabert K, Theodoridis G, Tsikas D (2020) GC-NICI-MS analysis of acetazolamide and other sulfonamide (R-SO2-NH2) drugs as pentafluorobenzyl derivatives [R-SO2-N(PFB)2] and quantification of pharmacological acetazolamide in human urine. Journal of Pharmaceutical Analysis 10(1): 49–59. https://doi.org/10.1016/j.jpha.2019.11.006[PubMed] [PMC]
Boyce M, Favela KA, Bonzo JA, Chao A, Lizarraga LE, Moody LR, Owens EO,
Patlewicz G, Shah I, Sobus JR, Thomas RS, Williams AJ, Yau A, Wambaugh JF (2023) Identifying xenobiotic metabolites with in silico prediction tools and LCMS
suspect screening analysis. Frontiers in Toxicology 5: 1051483. https://doi.org/10.3389/ftox.2023.1051483 [PubMed] [PMC]
Chen W-H, Chiu C-H, Farn S-S, Cheng K-H, Huang Y-R, Lee S-Y, Fang Y-C, Lin Y-H, Chang K-W (2023) Identification of the hepatic metabolites of flumazenil and their kinetic application in neuroimaging. Pharmaceuticals 16(5): 764. https://doi.org/10.3390/ph1605076 [PubMed] [PMC]
Dhandar AG, Chaudhari SR, Ganorkar SB, Patil AS, Surana SJ (2022) Mini-review on bioanalytical estimation of brinzolamide. Current Pharmaceutical Analysis 18(3): 265–272. https://doi.org/10.2174/1573412917666210812103414
1.2.1.1.0008.15 The Mass-spectrometry» (2023) The State Pharmacopoeia of Russian Federation. XV edition https://pharmacopoeia.regmed.ru/pharmacopoeia/izdanie-15/
1.2.1.2.0001.15 The Chromatography (2023) The State Pharmacopoeia of Russian Federation. XV edition https://pharmacopoeia.regmed.ru/pharmacopoeia/izdanie-15/
Khokhlov AL, Shetnev AA, Korsakov MK, Fedorov VN, Tyushina AN, Volkhin NN, Vdovichenko VP (2023) Pharmacological properties of sulfonamide derivatives – new inhibitors of carbonic anhydrase. Bulletin of Experimental Biology and Medicine 175(2): 166–170. https://doi.org/10.47056/0365-9615-2023-175-2-166-170 [PubMed]
Khokhlov AL, Yaichkov II, Korsakov MK, Shetnev AA, Volkhin NN, Petukhov SS (2023) Development of quantification methods of a new selective carbonic anhydrase II inhibitor in plasma and blood and study of the pharmacokinetics of its ophthalmic suspension in rats. Research Results in Pharmacology 9(4): 53–64. https://doi.org/10.18413/rrpharmacology.9.10056
Khokhlov AL, Yaichkov II, Shetnev AА, Ivanovsky SA, Korsakov MK, Alexeev MA, Gasilina OA, Volkhin NN, Petukhov SS (2024) Identification and synthesis of metabolites of the new antiglaucoma drug. Research Results in Pharmacology 10(1): 53–66. https://doi.org/10.18413/rrpharmacology.10.431
Kurysheva NI (2020) Carbonic anhydrase inhibitors in the treatment of glaucoma. Review. Part II. Ophthalmology in Russia [Oftal'mologiya] 17(4): 676–682. https://doi.org/10.18008/1816-5095-2020-4-676-682 [in Russian]
Lo Faro AF, Tini A, Gottardi M, Pirani F, Sirignano A, Giorgetti R, Busardò FP (2021) Development and validation of a fast ultra-high-performance liquid chromatography tandem mass spectrometry method for determining carbonic anhydrase inhibitors and their metabolites in urine and hair. Drug Testing and Analysis 13(8): 1552–1560. https://doi.org/10.1002/dta.3055 [PubMed] [PMC]
Peeters L, Vervliet P, Foubert K, Hermans N, Pieters L, Covaci A (2020) A comparative study on the in vitro biotransformation of medicagenic acid using human liver microsomes and S9 fractions. Chemico-Biological Interactions 328: 109192. https://doi.org/10.1016/j.cbi.2020.109192 [PubMed]
Reddy GN, Laltanpuii C, Sonti R (2021) Review on in vivo profiling of drug metabolites with LC-MS/MS in the past decade. Bioanalysis 13(22): 1697–1722. https://doi.org/10.4155/bio-2021-0144 [PubMed]
Simakova IL, Grigoryan LA, Gorbacheva KS (2023) Modern possibilities of functional glaucoma screening (Part 1). National Journal Glaucoma 22(4): 99–111. https://doi.org/10.53432/2078-4104-2023-22-4-99-111 [in Russian]
Trawinski J, Wronski M, Gawlik M, Skibinski R (2022) Identification of the new metabolite of nebivolol using liquid chromatography coupled with high-resolution mass spectrometry and chemometrics. Molecules 27(3): 763. https://doi.org/10.3390/molecules27030763[PubMed] [PMC]
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Khokhlov AL, Yaichkov II, Shetnev AA, Panova VA, Efimova YA, Ivanovskiy SA, Korsakov MK, Vol’khin NN, Petukhov SS

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

