Development of quantification methods of a new selective carbonic anhydrase II inhibitor in plasma and blood and study of the pharmacokinetics of its ophthalmic suspension in rats
DOI:
https://doi.org/10.18413/rrpharmacology.9.10056Abstract
Introduction: Development of new bioanalytical methods is required for studying the systemic exposure of new selective inhibitor of carbonic anhydrase II, 4-(2-methyl-1,3-oxazole-5-yl)-benzenesulfonamide, and its N-hydroxymetabolite in plasma and in whole blood. The results of the experiment with a single administration of an ophthalmic suspension of the drug are necessary to optimize the subsequent design of a full pharmacokinetic study.
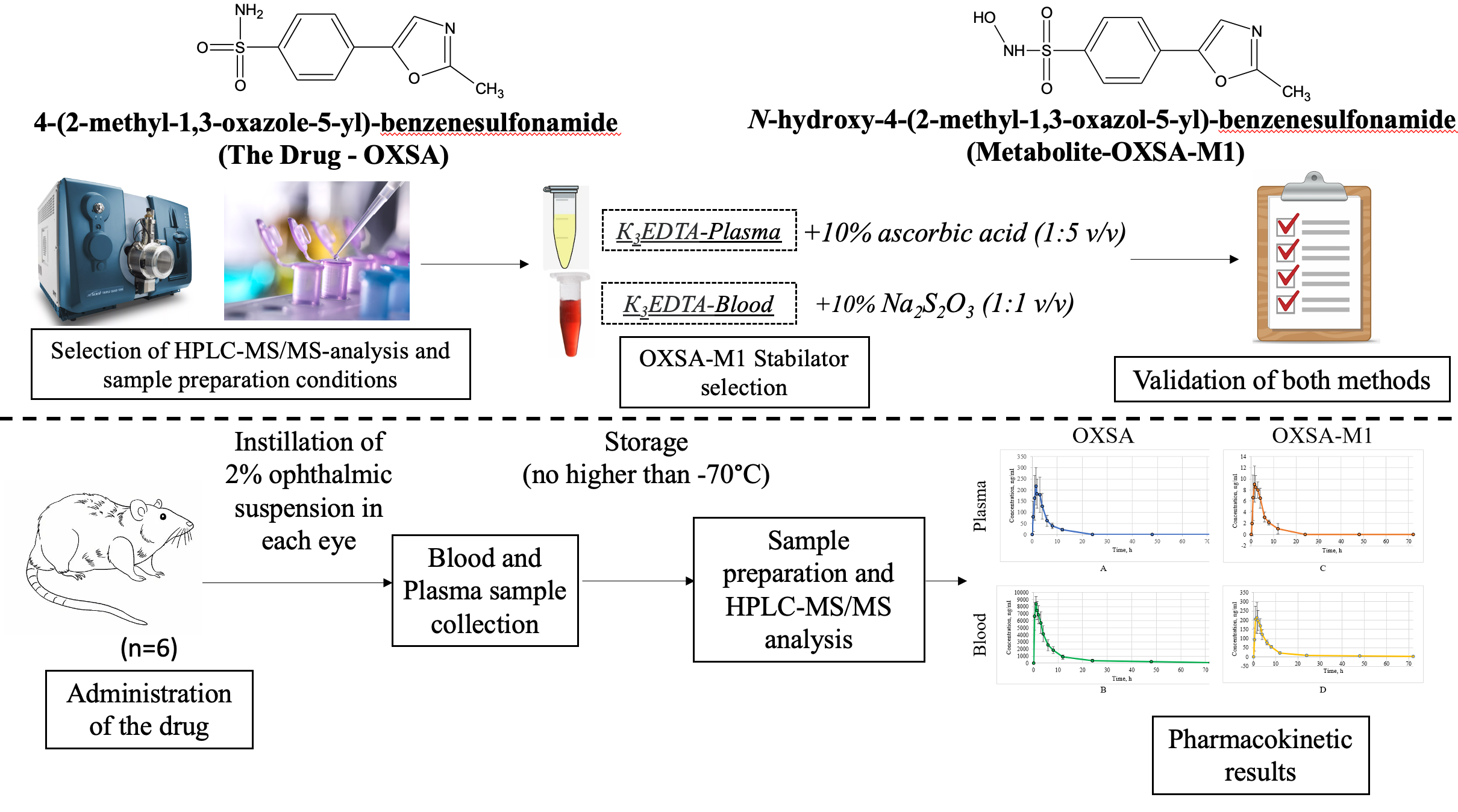
Materials and Methods: HPLC-MS/MS method was used to measure a concentration of analytes in plasma and whole blood. Chromatographic separation was performed on the Poroshell 120EC-C18 column (50*3.0 mm, 2.7 µm). Pharmacokinetics was studied on 6 Wistar rats weighing 287.50±18.64 g (Mean±SD). Each animal was instilled with 40 µL of the ophthalmic suspension in concentration of 2% in each eye. Blood samples were collected before administration of the drug and 30 min, 1 h, 1 h 30 min, 2 h, 3 h, 4 h, 6 h, 8 h, 12 h, 24 h, 48 h, and 72 h after administration. Non-compartment approach was used for the evaluation of pharmacokinetic parameters.
Results and Discussion: The protein precipitation was chosen for a sample preparation of biological fluids. A solution of ascorbic acid in concentration of 10% was added to plasma, and a solution of sodium thiosulfate in concentration of 10% was added to blood to prevent the degradation of N-hydroxymetabolite of the drug. The analytical range of determination of 4-(2-methyl-1,3-oxazole-5-yl)-benzenesulfonamide and its N-hydroxyderivative in blood was 50-10000 ng/mL and 5-1000 ng/mL, respectively, in plasma – 10-2000 ng/mL and 1-200 ng/mL, respectively. The maximum plasma concentration of the studied drug was 264.32±68.47 ng/mL (Mean±SD) 1.92±0.92 h (Mean±SD) after administration, and its metabolite was 10.43±1.79 ng/mL 2.17±1.13 h after administration. The maximum concentration of the drug in blood reached 8705.23±1301.84 ng/mL (Mean±SD) 1.17±0.52 h (Mean±SD) after administration, and the maximum concentration of N-hydroxymetabolite reached 230.00±69.54 ng/mL (Mean±SD) 1.33±0.41 h (Mean± SD) after administration.
Conclusion: The developed methods have been fully validated according to the requirements of Russian and internatonal guidelines and have been successfully used for pharmacokinetic research. It was found that a content of 4-(2-methyl-1,3-oxazole-5-yl)-benzenesulfonamide and its main metabolite in whole blood is significantly higher than in plasma.
Graphical Abstract
Keywords:
HPLC-MS/MS, stabilization, pharmacokinetics, selective carbonic anhydrase II inhibitorReferences
Ferraroni M, Lucarini L, Masini E, Korsakov M, Scozzafava A, Supuran CT, Krasavin M (2017) 1,3-Oxazole-based selective picomolar inhibitors of cytosolic human carbonic anhydrase II alleviate ocular hypertension in rabbits: Potency is supported by X-ray crystallography of two leads. Bioorganic & Medicinal Chemistry 25 (17): 4560–4565. https://doi.prg/10.1016/j.bmc.2017.06.054 [PubMed]
Foivas A, Malenović A, Kostić N, Božić M, Knežević M, Loukas YL, Dotsikas Y (2016) Quantitation of brinzolamide in dried blood spots by a novel LC-QTOF-MS/MS method. Journal of Pharmaceutical and Biomedical Analysis 119(5): 84–90. https://doi.org/10.1016/j.jpba.2015.11.043 [PubMed]
ICH Guideline M10 on Bioanalytical Method Validation and Study Sample Analysis (2022) https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m10-bioanalytical-method-validation-step-5_en.pdf. (access date: 15 May 2023)
Khokhlov AL, Yaichkov II, Dzhurko YuA, Shitov LN, Shitova AM (2018) Methodical approaches to bioassay of substances containing unstable functional groups. Research Results in Pharmacology 4(1): 33–42. https://doi.org/10.3897/rrpharmacology.4.25253
Kintz P, Gheddar L, Raul J-S (2022) Adverse analytical finding due to red blood cells transfusion: A rare case involving the diuretic dorzolamide. Drug Testing and Analysis 14(10): 1785–1790. https://doi.org/10.1002/dta.3342 [PubMed]
Lo Faro AF, Tini A, Gottardi M, Pirani F, Sirignano A, Giorgetti R, Busardò FP (2021) Development and validation of a fast ultra-high-performance liquid chromatography tandem mass spectrometry method for determining carbonic anhydrase inhibitors and their metabolites in urine and hair. Drug Testing and Analysis 13(8): 1552–1560. https://doi.org/10.1002/dta.3055[PubMed] [PMC]
Madrewar BA, Deshpande A, Bhattacharya S (2022) Mini-Review on bioanalytical estimation of brinzolamide. Current Pharmaceutical Analysis 18(3): 265–272. https://doi.org/10.2174/1573412917666210812103414
Mironov A N (ed.) (2012) Guidelines for Conducting Preclinical Studies of Medicines. Volume 1. Polygraph Plus, Moscow, 944 pp.
Mironov AN (2014) Guidance on Inspection of Medicines. Volume 1. Polygraph Plus, Moscow, 328 рp. [in Russian]
On Approval of the Rules for Conducting Bioequivalence Studies on Medicines in the Eurasian Economic Union. Decision of the Council of the Eurasian Economic Commission №85 of November 3, 2016 (2016) http://docs.cntd.ru/document/456026107 (access date: 15 May 2023).
Strakhov VV, Korsakov MK, Fedorov VN, Vdovichenko VP, Shetnev AA, Popova AA, Volkhin NN (2023) Carbonic anhydrase inhibitors for the treatment of glaucoma. Medical Ethics 1: 44–50. https://doi.org/10.24075/medet.2023.001
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Александр Л. Хохлов, Илья И. Яичков, Михаил К. Корсаков, Антон А. Шетнев, Никита Н. Вольхин, Сергей С. Петухов

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

