Experimental evaluation of osteogenic activity of certain hormonal drugs
DOI:
https://doi.org/10.18413/rrpharmacology.11.557Abstract
Introduction: The necessity of extra medicamental stimulation of bone tissue regeneration in addition to basic surgical method of treating fractures is usually determined by osteoporosis or high severity of injury. One of the lesser-studied options for osteogenic hormone therapy of fractures is the use of human chorionic gonadotropin(hCG). The aim of the study: Conducting an experimental evaluation of the osteogenic activity of hCG in its systemic and local application based on a model of perforated fracture of femoral bone in laboratory white rats.
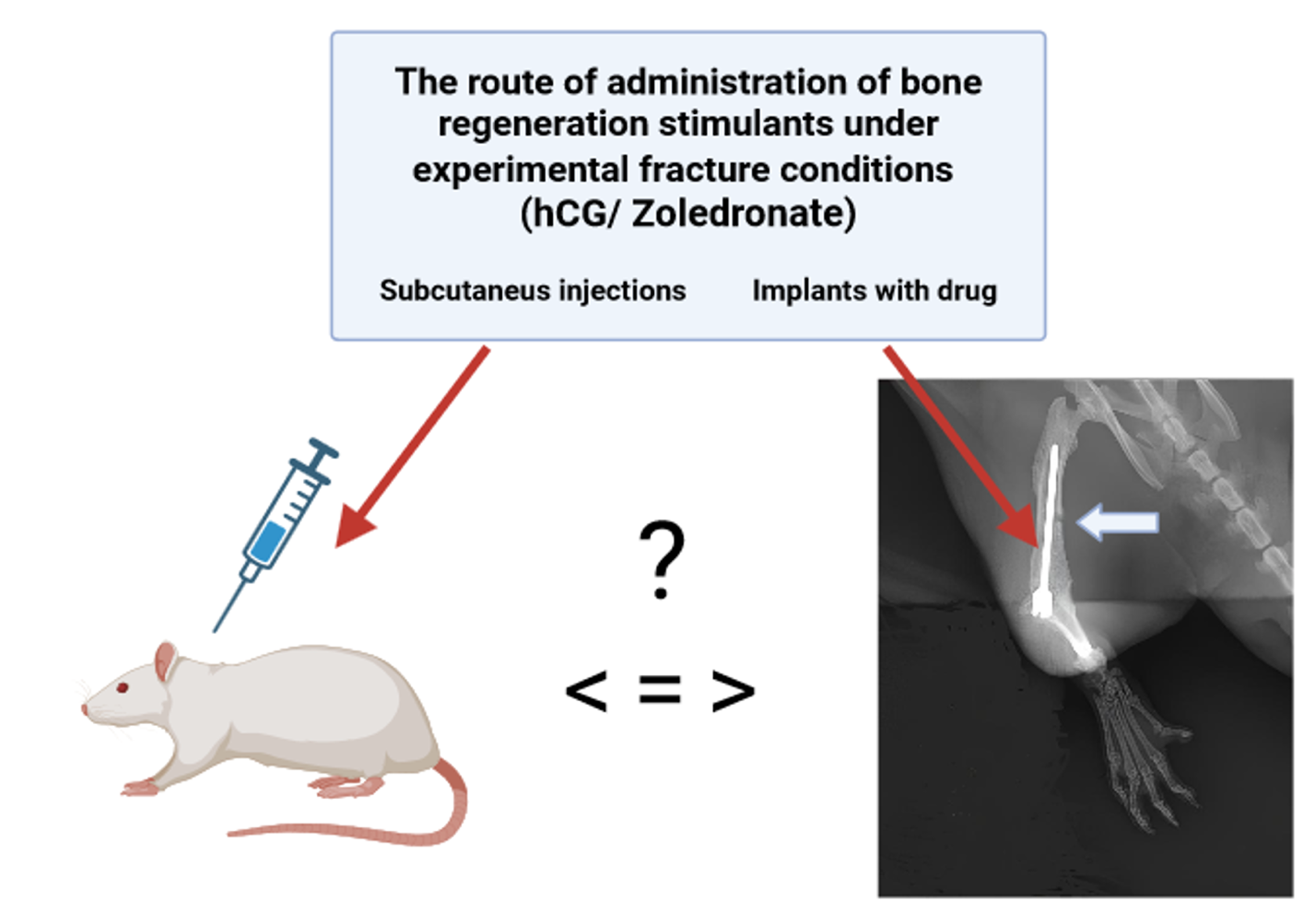
Materials and Methods: The experimental perforated femoral fracture in 64 male Wistar laboratory rats was modeled by drilling a circular bone defect with a 1.4 mm diameter. The osteogenic effect of the studied hormonal drugs (hCG and thyrocalcitonin) compared with a reference agent from the bisphosphonate group – zoledronic acid, after a course of injectable treatment was assessed according to the rate of bone defect recovery using digital radiography. In addition, the same method was used to experimentally evaluate the local osteogenic activity of hCGand zoledronate introduced into the bone marrow canal of the damaged bone via a patented original intramedullary metal construction for osteosynthesis.
Results and Discussion: Upon administering the studied hormonal drugs by injection, significant osteogenic activity was observed, with a more pronounced effect for hCG, although still inferior to that of the reference pharmacotherapy. The intra-bone local application of hCG showed results practically equal to the osteogenic effect of zoledronate.
Conclusion: As a result of the experiment, a high therapeutic effect of hCG on bone tissue regeneration was discovered, which, with local application (intramedullary implants soaked in hCG solution with a concentration of 5000 IU/mL), reached the level of zoledronate’s effect (intramedullary implants soaked in solution with a concentration of 0.8 mg/mL), but without the toxic side effects typically associated with the reference pharmacotherapy.
Graphical Abstract
Keywords:
bone fracture, osteoporosis, bone regeneration stimulators, human chorionic gonadotropin, thyrocalcitonin, zoledronate, rats, experimentReferences
Akimova TN, Savchenko VV, Gladkova EV, Kolmykova AS, Chibrikov AG (2009) Average duration of temporary disability in patients with long bone fractures. Trauma [Travma] 10(1): 44– [in Russian]
Belaya ZhE, Belova KYu, Biryukova EV, Dedov II, Dzeranova LK, Drapkina OM, Dreval AV, Dubovitskaya TA, Dudinskaya EN, Ershova OB, Zagorodniy NV, Ilyukhina OB, Kanis JA, Kryukova IV, Lesnyak OM, Mamedova EO, Marchenkova LA, Mel’nichenko GA, Nikankina LV, Nikitinskaya OA, Petryaikin AV, Pigarova EA, Rodionova SS, Rozhinskaya LYa, Skripnikova IA, Tarbaeva NV, Tkacheva ON, Toroptsova NV, Farba LYa, Tsoriev TT, Chernova TO, Yureneva SV, Yakushevskaya OV (2021) Federal clinical guidelines for diagnosis, treatment and prevention of osteoporosis. Osteoporosis and Bone Diseases [Osteoporoz i Osteopatii] 24(2): 4–47. https://doi.org/10.14341/osteo12930 [in Russian]
Black DM, Rosen CJ (2016) Clinical practice: Postmenopausal osteoporosis. New England Journal of Medicine374(3): 254–262. https://doi.org/10.1056/NEJMcp1513724 [PubMed]
Cole LA (2012) hCG, the wonder of today’s science. Reproductive Biology and Endocrinology 10: 24. https://doi.org/10.1186/1477-7827-10-24 [PubMed] [PMC]
Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, McClung M, Roux C, Törring O, Valter I, Wang AT, Brown JP (2018) Vertebral fractures after discontinuation of denosumab: A post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. Journal of Bone and Mineral Research33(2): 190–198. https://doi.org/10.1002/jbmr.3337 [PubMed]
Mansell JP, Bailey AJ, Yarram SJ (2007) Could bone tissue be a target for luteinizing hormone/chorionic gonadotropin? Molecular and Cellular Endocrinology 269(1–2): 99–106 https://doi.org/10.1016/j.mce.2006.06.016 [PubMed]
Okladnikov SM, Nikitina SY (Eds) (2023) Healthcare in Russia. Statistical compendium. Rosstat, Moscow, 179 pp. [in Russian]
Tucker J (2006) Stimulation of proliferation of pluripotential stem cells through administration of pregnancy-associated compounds. US Patent 20060089309 A1. USA.
Smirnov NA, Koryshkov NA, Shcherbakov AO, Volkhin NN, Rusanov NO, Kurchinsky DD, Dvoretsky DA (2019) Metal construction for intramedullary osteosynthesis of tubular bones. Russian Federation Patent 187285 U1. IPC A61B 17/72. Application No. 2019101171. Filed 17 January 2019. Published 28 February 2019. Applicant: LLC ”Demiko-pro”. [in Russian]
Vahle JL, Long GG, Sandusky G, Westmore M, Ma YL, Sato M (2004) Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicologic Pathology 32(4): 426–438. https://doi.org/10.1080/01926230490462138 [PubMed]
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Smirnov NA, Volkhin NN, Khokhlov AL, Bondarev RA, Smirnov RR

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

