The EPOR/CD131 heteroreceptoragonist has an endothelioprotective effect against the background of pulmonary hypertension caused by monocrotalin
DOI:
https://doi.org/10.18413/rrpharmacology.9.10057Abstract
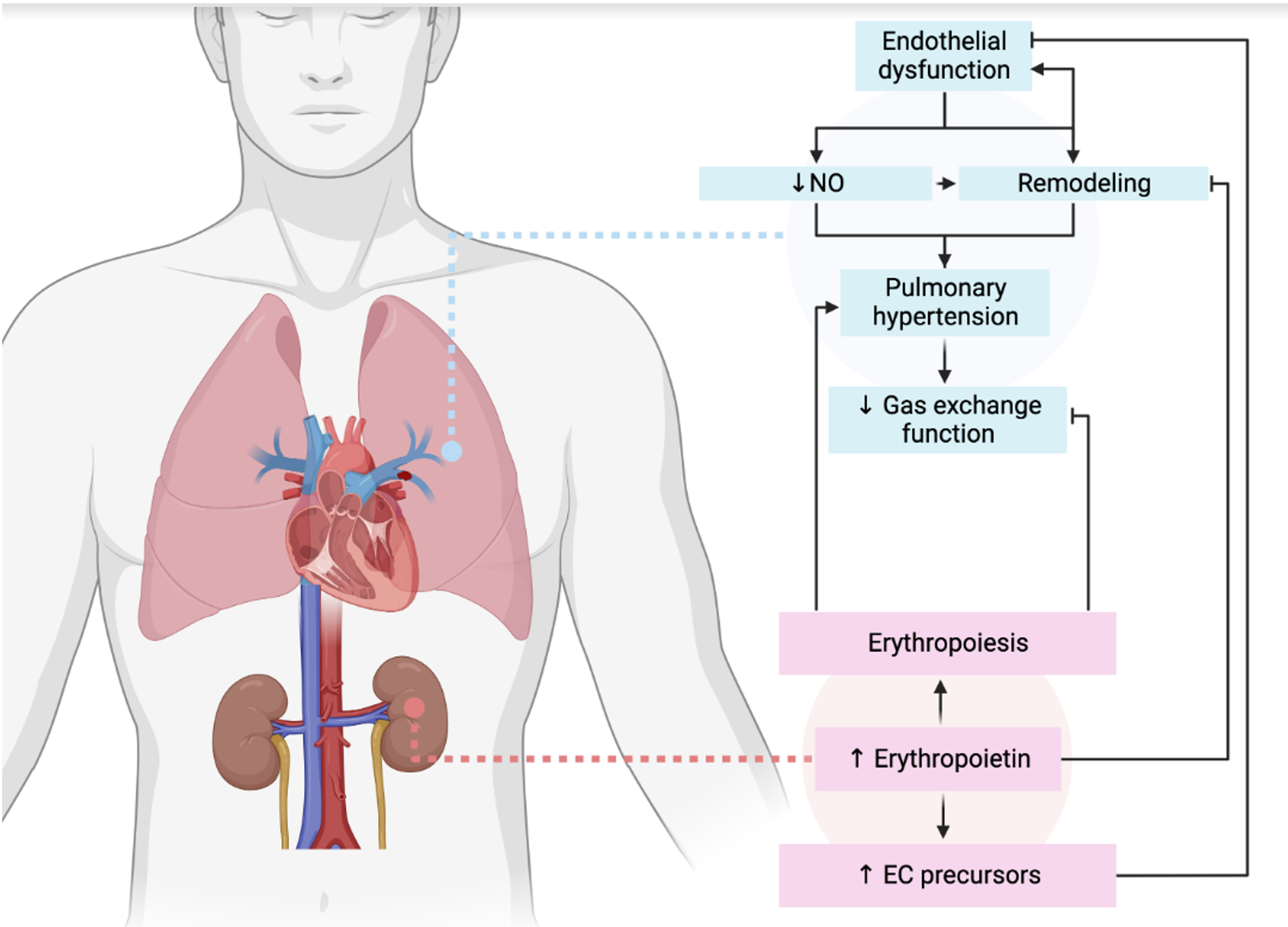
Introduction: The abnormal increase in pulmonary pressure observed in pulmonary arterial hypertension (PAH) is a consequence of increased pulmonary vascular resistance due to progressive loss and obliteration of pulmonary arteries. The initial trigger is a combination of factors that lead to endothelial damage and impaired vascular regeneration. Aim: research the possibilities of pharmacological correction of pulmonary arterial hypertension induced by monocrotalin using the EPOR/CD131 heteroreceptor agonist with the laboratory code EP-11-3.
Materials and Methods: The study of pharmacological activity on a model of monocrotaline induced PAH was carried out on male Sprague-Dawley rats weighing 180-220 grams. Monocrotaline (MCT) pulmonary hypertension was simulated in 30 animals using subcutaneous injection of MCT at a dose of 60 mg/kg. Seven days after the injection of MCT, the administration of the studied compounds began. The erythropoietin derivative with the laboratory code EP-11-3 and pHBSP administered subcutaneously at a dose of 25 mcg/kg once every 3 days for 21 days.
Results: On the model of monocrotalin-induced PAH, it was shown that the erythropoietin derivative with the laboratory code EP-11-3 has a pronounced endothelioprotective effect, reducing the coefficient of endothelial dysfunction, statistically significantly increasing the expression of VEGF-R2 mRNA and reducing the expression of SDF-1 mRNA, reducing the concentrations of CT-1 and PNP, and reducing the signs of remodeling of the heart and pulmonary vessels.
Conclusion: Erythropoietin derivative with laboratory code EP-11-3 has an endothelioprotective effect and reduces the manifestations of vascular remodeling in pulmonary hypertension caused by monocrotalin.
Graphical Abstract
Keywords:
Monocrotaline, EPOR/CD131, progenitors of endothelial cells, endothelioprotector, pulmonary hypertensionReferences
Brines M, Patel NS, Villa P, Brines C, Mennini T, De Paola M, Erbayraktar Z, Erbayraktar S, Sepodes B, Thiemermann C, Ghezzi P, Yamin M, Hand CC, Xie QW, Coleman T, Cerami A (2008) Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proceedings of the National Academy of Sciences of the United States of America 105(31): 10925–10930. https://doi.org/10.1073/pnas.0805594105 [PubMed] [PMC]
Golubev IV, Gureev VV, Korokin MV, Zatolokina MA, Avdeeva EV, Gureeva AV, Rozhkov IS, Serdyuk EA, Soldatova VA (2020) Preclinical study of innovative peptides mimicking the tertiary structure of the α-helix B of erythropoietin. Research Results in Pharmacology 6(2): 85–96. https://doi.org/10.3897/rrpharmacology.6.55385
Ikarashi N, Toba K, Kato K, Ozawa T, Oda M, Takayama T, Kobayashi H, Yanagawa T, Hanawa H, Suzuki T, Nakazawa M, Nomoto M, Asami F, Higuchi M, Saito H, Aizawa Y (2012) Erythropoietin, but not asialoerythropoietin or carbamyl-erythropoietin, attenuates monocrotaline-induced pulmonary hypertension in rats. Clinical and Experimental Hypertension 34(8): 575–581.https://doi.org/10.3109/10641963.2012.681728 [PubMed]
Karamanian VA, Harhay M, Grant GR, Palevsky HI, Grizzle WE, Zamanian RT, Ihida-Stansbury K, Taichman DB, Kawut SM, Jones PL (2014) Erythropoietin upregulation in pulmonary arterial hypertension. Pulmonary Circulation 4(2): 269–279. https://doi.org/10.1086 /675990 [PubMed] [PMC]
Kurakula K, Smolders VFED, Tura-Ceide O, Jukema JW, Quax PHA, Goumans MJ (2021) Endothelial dysfunction in pulmonary hypertension: Cause or consequence? Biomedicines 9(1): 57. https://doi.org/10.3390/biomedicines9010057 [PubMed] [PMC]
Kuzubova EV, Radchenko AI, Pokrovsky VM (2022) Pathological conditions associated with tau protein: mechanisms of development and possible biological targets for pharmacological correction of tau proteinopathy (review). Research Results in Biomedicine 8(4): 474–494. https://doi.org/10.18413/2658-6533-2022-8-4-0-5
McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon MD, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J (2009) ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. Journal of the American College of Cardiology 53(17): 1573–1619. https://doi.org/10.1161/CIRCULATIONAHA.109.192230[PubMed]
Peng B, Kong G, Yang C, Ming Y (2020) Erythropoietin and its derivatives: from tissue protection to immune regulation. Cell Death & Disease 11(2): 79. https://doi.org/10.1038/s41419-020-2276-8 [PubMed] [PMC]
Pompilio G, Capogrossi MC, Pesce M, Alamanni F, DiCampli C, Achilli F, Germani A, Biglioli P (2009) Endothelial progenitor cells and cardiovascular homeostasis: clinical implications. International Journal of Cardiology 131(2): 156–167. https://doi.org/10.1016/j.ijcard.2008.08.033 [PubMed]
Puchenkova OA, Nadezhdin SV, Soldatov VO (2020) Study of antiatherosclerotic and endothelioprotective activity of peptide agonists of epor/cd131 heteroreceptor. Pharmacy & Pharmacology 8(2): 100–111. https://doi.org/10.19163/2307-9266-2020-8-2-100-111
Ranchoux B, Harvey LD, Ayon RJ, Babicheva A, Bonnet S, Chan SY, Yuan JX, Perez VJ (2018) Endothelial dysfunction in pulmonary arterial hypertension: an evolving landscape. Pulmonary Circulation 8(1): 2045893217752912. https://doi.org/10.1177/2045893217752912 [PubMed] [PMC]
Rubin LJ (2006) Pulmonary arterial hypertension. Proceedings of the American Thoracic Society 3(1): 111–115. https://doi.org/10.1513/pats.200510-112JH [PubMed]
Satoh K, Kagaya Y, Nakano M, Ito Y, Ohta J, Tada H, Karibe A, Minegishi N, Suzuki N, Yamamoto M, Ono M, Watanabe J, Shirato K, Ishii N, Sugamura K, Shimokawa H (2006) Important role of endogenous erythropoietin system in recruitment of endothelial progenitor cells in hypoxia-induced pulmonary hypertension in mice. Circulation 113(11): 1442–1450.https://doi.org/1Q19x3wmkRsfrHjcC5MuLgjGds733ApvwS [PubMed]
Urakami T, Järvinen TA, Toba M, Sawada J, Ambalavanan N, Mann D, McMurtry I, Oka M, Ruoslahti E, Komatsu M (2011) Peptide-directed highly selective targeting of pulmonary arterial hypertension. The American Journal of Pathology 178(6): 2489–2495. https://doi.org/10.1016/j.ajpath.2011.02.032 [PubMed] [PMC]
van Loon RL, Bartelds B, Wagener FA, Affara N, Mohaupt S, Wijnberg H, Pennings SW, Takens J, Berger RM (2015) Pulmonary vascular remodeling in experimental pulmonary arterial hypertension through interplay between endothelial progenitor cells and heme oxygenase. Frontiers in Pediatrics 3: 71. https://doi.org/10.3389/fped.2015.00071 [PubMed] [PMC]
YanYun P, Wang S, Yang J, Chen B, Sun Z, Ye L, Zhu J, Wang X (2015) Interruption of CD40 Pathway improves efficacy of transplanted endothelial progenitor cells in monocrotaline induced pulmonary arterial hypertension. Cellular Physiology and Biochemistry 36(2): 683–696. https://doi.org/ 10.1159/000430130 [PubMed]
Zagrebelnaya AV, Korokina LV, Malorodova TN, Shcheblykina OV, Avtina TV, Bolgov AА, Malyutina ES, Simonov VI, Travkin VA, Dmitriev AА, Aripov AA, Shcheblykin DV (2023) Pulmonary hypertension: modern methods of treatment and ways of their long-term development. Research Results in Pharmacology 9(2): 37–54. https://doi.org/10.18413/rrpharmacology.9.10026
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Lilia V. Korokina

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

