Investigation of the pharmacological activity of the tetrapeptide HAEЕ, zinc, and human serum albumin in a transgenic mouse model with tau protein overexpression (P301S)
DOI:
https://doi.org/10.18413/rrpharmacology.11.493Abstract
Introduction: Neurodegenerative diseases affecting neurons represent a wide spectrum of pathological conditions. To slow the accumulation of pathological tau protein, therapeutic interventions such as a pharmaceutical composition consisting of the tetrapeptide HAEЕ, zinc, and albumin may be employed. This composition possesses properties that can potentially slow disease progression.
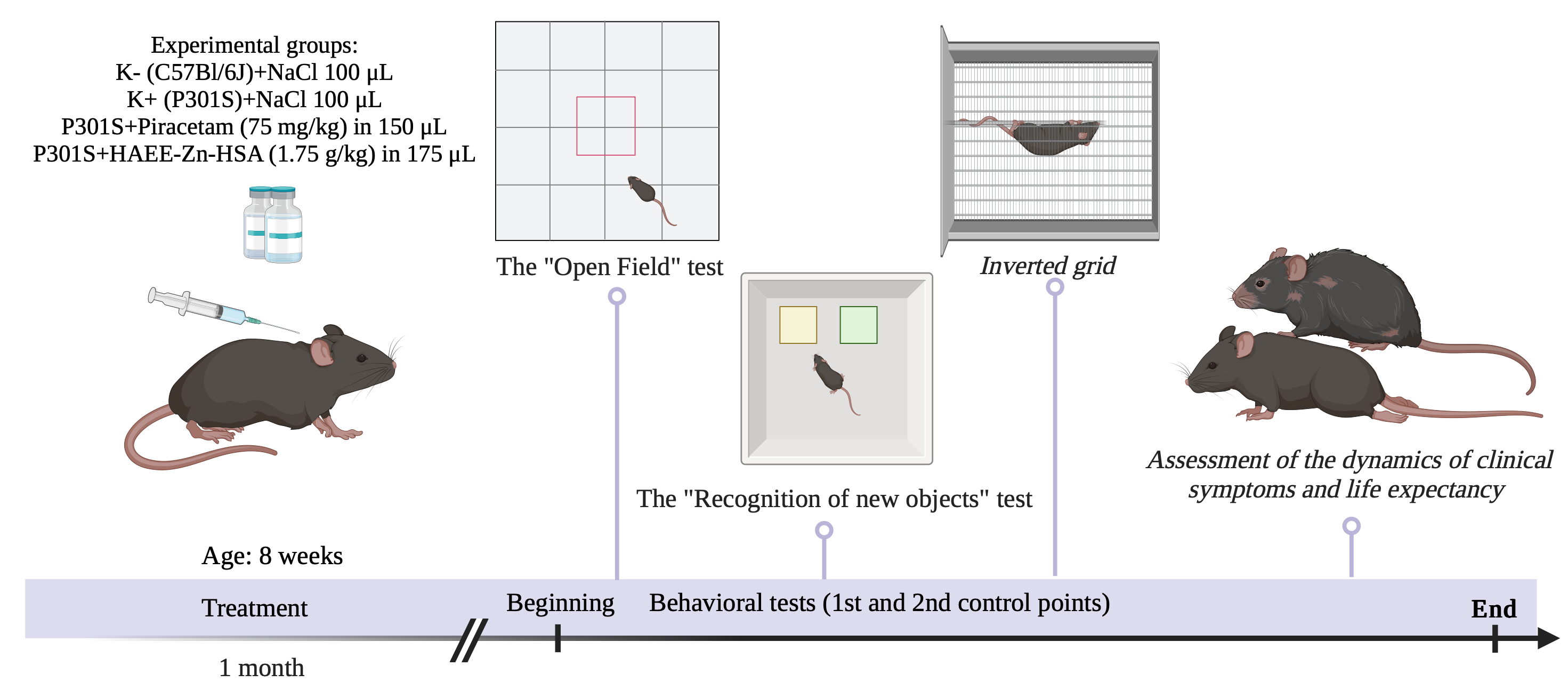
Materials and Methods: The experiment was performed on 60 homozygous mice of both sexes from a transgenic line with the overexpression of human mutant tau protein (P301S) and on 20 mice from a wild-type C57Bl/6J background line. Control groups (C57Bl/6J – K and P301S – K+) received NaCl injection water (100 µL subcutaneously, once every 2 days); the experimental group (P301S) received HAEE-Zn-HSA (75 mg/kg, 150 µL subcutaneously, once every 2 days); and the comparison group (P301S) received piracetam (1.75 g/kg, 175 µL subcutaneously, once every 2 days). Behavioral activity was analyzed using tests at two time points, and the phenotypic presentation and lifespan were evaluated.
Results and Discussion: The P301S group of mice treated with HAEE-Zn-HSA at a dosage of 75 mg/kg showed positive behavioral activity in the following tests: Open Field, Novel Object Recognition, and Inverted grid test, indicating increased exploratory activity, and improved motor function and coordination in the mice of this group. Pharmacological intervention with HAEE-Zn-HSA in the experimental group of transgenic mice demonstrated positive results, as a statistically significant effect on lifespan and delayed onset of the phenotypic presentation of the disease was shown (24±2.14 weeks, p≤0.05).
Conclusion: The pharmaceutical composition HAEE-Zn-HSA at a dosage of 75 mg/kg, using a model of homozygous mice from a transgenic line with overexpression of human mutant tau protein P301S, demonstrated high levels of adaptive and exploratory activity and improved motor function.
Graphical Abstract
Keywords:
tauopathy, tau protein, behavioral activity, transgenic mice, HAEE-Zn-HSA complex pharmaceutical compositionReferences
Alquezar C, Arya S, Kao AW (2021) Tau Post-translational modifications: Dynamic transformers of tau function, degradation, and aggregation. Frontiers in Neurology 11: 595532. https://doi.org/10.3389/fneur.2020.595532 [PubMed] [PMC]
Barykin EP, Garifulina AI, Tolstova AP, Anashkina AA, Adzhubei AA, Mezentsev YV, Shelukhina IV, Kozin SA, Tsetlin VI, Makarov AA (2020) Tetrapeptide Ac-HAEE-NH2 protects α4β2 nAChR from inhibition by Aβ. International Journal of Molecular Sciences 21(17): 6272. https://doi.org/10.3390/ijms21176272 [PubMed] [PMC]
Deykin AV, Shcheblykina OV, Povetka EE, Golubinskaya PA, Pokrovskiy VM, Korokina LV, Vanchenko OA, Kuzubova EV, Trunov KS, Vasyutkin VV, Radchenko AI, Danilenko AP, Stepenko JV, Kochkarova IS, Belyaeva VS, Yakushev VI (2021) Genetically modified animals for use in biopharmacology: from research to production. Research Results in Pharmacology 7(4): 11–27. https://doi.org/10.3897/rrpharmacology.7.76685 [in Russian]
Hurtle B, Donnelly CJ, Zhang X, Thathiah A (2024) Live-cell visualization of tau aggregation in human neurons. Communications Biology 7(1): 1143. https://doi.org/10.1038/s42003-024-06840-z [PubMed] [PMC]
Kozin SA, Morozov AO, Chuev VP (2019) Pharmaceutical composition based on HAEE peptide for the treatment of neurodegenerative diseases: patent 2709539 Russian Federation: IPC A61K 31/315 (2006.01), A61K 38/07 (2006.01), A61K 38/38 (2006.01), A61P 25/28 (2006.01); patent holder JSC Pilot-Experimental Plant VladMiVa. – No. 2019125750, appl. 2019-08-15, publ. 2019-12-18, Bull. No. 35. – 16 p. [in Russian]
Muralidar S, Ambi SV, Sekaran S, Thirumalai D, Palaniappan B (2020) Role of tau protein in Alzheimer’s disease: The prime pathological player. International Journal of Biological Macromolecules 163: 1599–1617. https://doi.org/10.1016/j.ijbiomac.2020.07.327 [PubMed]
Park S, Shin J, Kim K, Kim D, Lee WS, Lee J, Cho I, Park IW, Yoon S, Lee S, Kim HY, Lee JH, Hong KB, Kim Y (2024) Modulation of amyloid and tau aggregation to alleviate cognitive impairment in a transgenic mouse model of Alzheimer’s disease. ACS Pharmacology and Translatiinal Science 7(9): 2650–2661. https://doi.org/10.1021/acsptsci.4c00006[PubMed]
Samudra N, Lane-Donovan C, VandeVrede L, Boxer AL (2023) Tau pathology in neurodegenerative disease: disease mechanisms and therapeutic avenues. The Journal of Clinical Investigation 133(12): e168553. https://doi.org/10.1172/JCI168553 [PubMed] [PMC]
Sindi G, Ismael S, Uddin R, Slepchenko KG, Colvin RA, Lee D (2024) Endogenous tau released from human ReNCell VM cultures by neuronal activity is phosphorylated at multiple sites. Preprint. bioRxiv. 597022. https://doi.org/10.1101/2024.06.02.597022 [PubMed] [PMC]
Stepenko YV, Shmigerova VS, Kostina DA, Shcheblykina OV, Zhernakova NI, Solin AV, Koroleva NV, Markovskaya VA, Dudnikova OV, Bolgov AA (2024) Study of the neuroprotective properties of the heteroreceptor EPOR/CD131 agonist of peptide structure in tau-proteinopathy modeling. Research Results in Pharmacology 10(2): 41–47. https://doi.org/10.18413/rrpharmacology.10.492 [in Russian]
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Veronika S. Shmigerova, Yulia V. Stepenko, Alexandra A. Kurbatova, Nikita S. Zhunusov, Nikita S. Lyapkalov, Maria S. Sviridova, Tatyana V. Avtina, Arkadiy V. Nesterov, Alexander A. Popov, Natalia I. Nesterova, Mariia Iu. Goltsova, Tatiana G. Pokrovskaya

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

