Features of bone remodeling and osteoreparation processes in modeling femoral fracture in genetically modified mice with impaired enzymatic regulation of steroid hormone metabolism
DOI:
https://doi.org/10.18413/rrpharmacology.9.10062Abstract
Introduction: The aim of this study was to evaluate the processes of bone remodeling and osteoreparation in modeling femoral fracture in mice with zero expression of 11β-HSD2 (11β-HSD2-/-) or both 11β-HSD2 and apolipoprotein e (11β-HSD2-/-/ApoE-/-).
Materials and Methods: The experimental study was conducted on 60 male mice weighing 24-30 g. The study used male mice that lacked the expression of 11β-HSD2 (knockout mice with the Hsd2-/- genotype) and male mice that lacked the expression of 11β-HSD2 and apolipoprotein E (double knockout mice with the Hsd2-/-/ApoE-/- genotype). The control group includes wild type C57bl/6 animals. Modeling of a fracture of the proximal metaphysis of the femur was performed in animals at the age of 6 months using a closed technique. Fracture fusion and bone remodeling and osteoreparation processes were evaluated 6 weeks after fracture modeling.
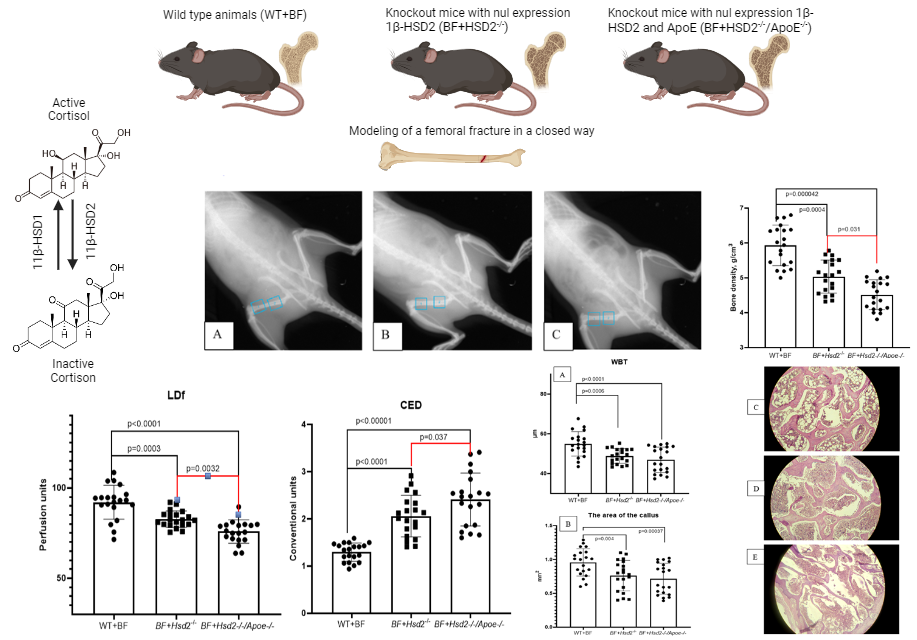
Results and Discussion: It has been shown that a violation of the regulation of steroid hormone metabolism in groups of animals with the Hsd2-/- and Hsd2-/-/ApoE-/- genotypes leads to an increase in the number of ungrown fractures by 3 and 3.5 times, respectively, in comparison with wild-type animals. It was found that the microcirculation level of the proximal metaphysis of the left femur in the area of the formed bone callus in the group of animals with the genotype 11β-HSD2-/- significantly decreased from 92.075±4.33 perfusion units (PE) in the group of wild-type animals to 82.67±3.54 PE (p=0.0002) in the group of animals with the genotype HSD2-/- and up to 75.85±5.64 (p<0.0001) in the group of mice with the Hsd2-/-/ApoE-/- genotype. When calculating the coefficient of endothelial dysfunction, an increase in the coefficient of endothelial dysfunction was found from 1.297±0.19 in intact animals to 2.115±0.45 (p<0.00001) in the group of mice with the Hsd2-/- genotype and to 2.41±0.04 (p<0.00001) in the group of mice with the Hsd2-/-/ApoE-/- genotype. In animals with impaired cortisol metabolism, there was a slowdown in the formation of bone tissue in the fracture area, bone trabeculae had a smaller width, large amounts of fibrous and connective tissue were observed in the lumen between bone fragments, and an increase in intertrabecular spaces was noted
Conclusion: The close relationship between the metabolism of 11β-HSD2 and NO, confirmed in this study, can be considered as a promising pharmacotherapeutic target. It is obvious that approaches to changing the activity of 11β-HSD have significant therapeutic potential in the treatment of osteoporosis, bone remodeling disorders and osteoreparation in fractures against the background of formed osteoporotic changes in the violation of steroid hormone metabolism.
Graphical Abstract
Keywords:
osteoporosis, bone density, steroid hormone metabolism, transgenic animals, 11ß-HSD2, ApoE, Hsd2-/-, Hsd2-/-/Apoe-/-References
Ballane G, Cauley JA, Luckey MM, Fuleihan Gel-H (2014) Secular trends in hip fractures worldwide: Opposing trends East versus West. Journal of Bone and Mineral Research 29(8): 1745–1755. https://doi.org/10.1002/jbmr.2218 [PubMed]
Camacho PM, Petak SM, Binkley N, Diab DL, Eldeiry LS, Farooki A, Harris ST, Hurley DL, Kelly J, Lewiecki EM, Pessah-Pollack R, McClung M, Wimalawansa SJ, Watts NB (2020) American Association of Clinical Endocrinologists / American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis – 2020 update executive summary. Endocrine Practice 26(5): 564–570. https://doi.org/10.4158/GL-2020-0524 [PubMed]
de Souza MP (2010) Osteoporosis diagnosis and treatment. Revista Brasileira de Ortopedia 45(3): 220–229. [PubMed] [PMC]
Deuchar GA, McLean D, Hadoke PWF, Brownstein DG, Webb DJ, Mullins JJ, Chapman K, Seckl JR, Kotelevtsev YV (2011) 11β-hydroxysteroid dehydrogenase type 2 deficiency accelerates atherogenesis and causes proinflammatory changes in the endothelium in apoe-/- mice. Endocrinology 152(1): 236–246. https://doi.org/10.1210/en.2010-0925 [PubMed] [PMC]
Dube S, Norby BJ, Pattan V, Carter RE, Basu A, Basu R (2015) 11β-hydroxysteroid dehydrogenase types 1 and 2 activity in subcutaneous adipose tissue in humans: Implications in obesity and diabetes. The Journal of Clinical Endocrinology and Metabolism 100(1): E70–E76. https://doi.org/10.1210/jc.2014-3017 [PubMed] [PMC]
Engström A, Michaëlsson K, Vahter M, Julin B, Wolk A, Åkesson A (2019) Associations between dietary cadmium exposure and bone mineral density and risk of osteoporosis and fractures among women. Bone 50(6): 1372–1378. https://doi.org/10.1016/j.bone.2012.03.018 [PubMed]
Korokin MV, Gudyrev OS, Lebedev PR, Kuzubova EV, Radchenko AI, Koklin IS, Taran EI, Kochkarov AA (2022a) Characteristics of the state of bone tissue in genetically modified mice with impaired enzymatic regulation of steroid hormone metabolism. Research Results in Pharmacology 8(4): 157–166. https://doi.org/10.3897/rrpharmacology.8.98779
Korokin MV, Soldatov VO, Gudyrev OS (2022b) The role of cortisol metabolism in the realization of pathogenetic links in the development of osteoporosis – the rationale for the search for new pharmacotherapeutic targets (review). Research Results in Biomedicine. 8(4): 457–473. [in Russian]
Lou S, Lv H, Yin P, Li Z, Tang P, Wang Y (2019) Combination therapy with parathyroid hormone analogs and antiresorptive agents for osteoporosis: A systematic review and meta-analysis of randomized controlled trials. Osteoporosis International 30(1): 59–70. https://doi.org/10.1007/s00198-018-4790-4 [PubMed]
Nogués X, Carbonell MC, Canals L, Lizán L, Palacios S (2022) Current situation of shared decision making in osteoporosis: A comprehensive literature review of patient decision aids and decision drivers. Health Science Reports 5(6): e849. https://doi.org/10.1002/hsr2.849 [PubMed] [PMC]
Rozenberg S, Bruyère O, Bergmann P, Cavalier E, Gielen E, Goemaere S, Kaufman JM, Lapauw B, Laurent MR, De Schepper J, Body JJ (2020) How to manage osteoporosis before the age of 50. Maturitas 138: 14–25. https://doi.org/10.1016/j.maturitas.2020.05.004 [PubMed]
Tabatabaei-Malazy O, Salari P, Khashayar P, Larijani B (2017) New horizons in treatment of osteoporosis. Daru 25(1): 2. https://doi.org/10.1186/s40199-017-0167-z [PubMed] [PMC]
Vidal M, Thibodaux RJ, Neira LFV, Messina OD (2019) Osteoporosis: A clinical and pharmacological update. Clinical Rheumatology 38(2): 385–395. https://doi.org/10.1007/s10067-018-4370-1 [PubMed]
Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O'Karma M, Wallace TC, Zemel BS (2016) The national osteoporosis foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporosis International (2016) 27: 1281–1386. https://doi.org/10.1007/s00198-015-3440-3 [PubMed] [PMC]
Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O'Brien CA, Thostenson J, Roberson PK, Boskey AL, Clemens TL, Manolagas SC (2010) Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in aged mice. Aging Cell 9(2): 147–161. https://doi.org/10.1111/j.1474-9726.2009.00545.x [PubMed] [PMC]
Zhang H, Zhou F, Pan Z, Bu X, Wang Y, Chen F (2017) 11β-hydroxysteroid dehydrogenases-2 decreases the apoptosis of MC3T3/MLO-Y4 cells induced by glucocorticoids. Biochemical and Biophysical Research Communications 490(4): 1399–1406. https://doi.org/10.1016/j.bbrc.2017.07.046 [PubMed]
Zhao B, Xing G, Wang A (2019) The BMP signaling pathway enhances the osteoblastic differentiation of bone marrow mesenchymal stem cells in rats with osteoporosis. Journal of Orthopaedic Surgery and Research 14(1): 462. https://doi.org/10.1186/s13018-019-1512-3 [PubMed] [PMC]
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Mikhail V. Korokin, Oleg S. Gudyrev, Petr R. Lebedev, Alim A. Kochkarov, Tatiana G. Pokrovskaya, Lyudmila M. Danilenko, Nina I. Zhernakova, Tatyana V. Avtina, Sergey A. Pribylov, Alina V. Parshina, Elena V. Kuzubova, Alexandra I. Radchenko, Ivan S. Koklin, Eduard I. Taran

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

