The influence of exogenous recombinant HSP 70 on the alteration of membrane stiffness in hippocampal neurons following the modeling of neonatal hypoxic-ischemic injury in mice
DOI:
https://doi.org/10.18413/rrpharmacology.10.547Abstract
Introduction: The use of atomic force microscopy (AFM) to investigate membrane stiffness in neurons provides valuable insights into cellular mechanisms and their alterations in response to various pathophysiological conditions. Heat shock protein HSP 70, a component of the cellular stress response system, plays a role in stabilizing the protein structures of cellular organelles. However, studies examining changes in the stiffness of hippocampal neuronal membranes in its presence, particularly following cerebral circulation disturbances, have not been conducted yet.
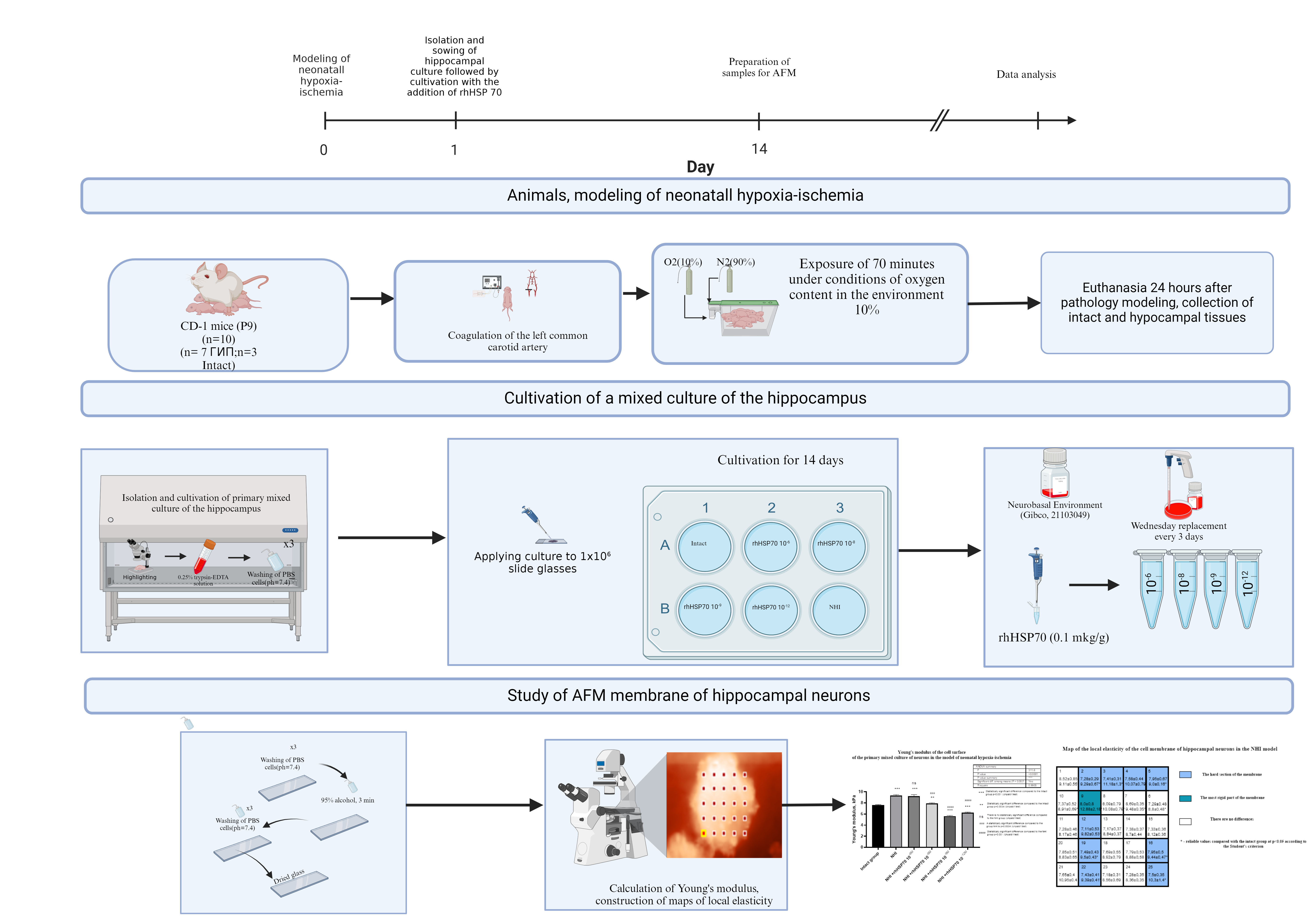
Materials and Methods: The study was performed on a mixed culture of hippocampal neurons derived from 9-day-old male CD-1 mice, obtained 24 hours after modeling neonatal hypoxia-ischemia. The following groups were formed: Intact culture; HI culture; HI + rhHSP70 10-6 M; HI + rhHSP70 10-8 M; HI + rhHSP70 10-9 M; HI + rhHSP70 10-12 M, with the substance added in dilutions from an initial dose of 0.1 µg/g. The Young's modulus was measured using force spectroscopy, and maps of local stiffness of various surface areas were generated.
Results and Discussion: The neonatal hypoxia-ischemia model resulted in an 18% increase in the stiffness of the neuronal cell surface compared to the control group (p<0.001). The addition of rhHSP70 at concentrations of 10-6 M and 10-8 M to the HI culture led to an increase in membrane stiffness by 20% (p<0.001) and 3% (p<0.0034), respectively, while dilutions of rhHSP70 at 10-9 M and 10-12 M resulted in a decrease in membrane stiffness by 35% (p<0.001) and 22% (p<0.001) compared to the intact group, respectively. In comparison to such in the neuronal culture group after neonatal hypoxia-ischemia modeling, membrane stiffness with the addition of rhHSP70 at 10-8 M, 10-9 M, and 10^-12 M decreased by 17% (p<0.0004), 65% (p<0.001), and 49% (p<0.001), respectively.
Conclusion: Thus, the addition of rhHSP 70 results in a reduction in membrane stiffness in the mixed culture of hippocampal neurons in mice, compared to the intact culture obtained after neonatal hypoxia-ischemia. The AFM method allows for the assessment of how various molecules, such as heat shock proteins (e.g., rhHSP70), influence the mechanical properties of membranes, which may be critically important for the development of new therapeutic agents.
Graphical Abstract
Keywords:
HSP 70, fetal hypoxia-ischemia, Young's modulus, hippocamp neuron membrane, AFMReferences
Belenichev IF, Aliyeva OG, Popazova OO, Bukhtiyarova NV (2023) Involvement of heat shock proteins HSP70 in the mechanisms of endogenous neuroprotection: the prospect of using HSP70 modulators. Frontiers in Cellular Neuroscience 17(1): 113–168. https://doi.org/10.3389/fncel.2023.1131683 [PubMed] [PMC]
Bryniarska-Kubiak N, Kubiak A, Trojan E, Wesołowska J, Lekka M, Basta-Kaim A (2023) Oxygen-glucose deprivation in organotypic hippocampal cultures leads to cytoskeleton rearrangement and immune activation: link to the potential pathomechanism of ischemic stroke. Cells 12: 1465. https://doi:10.3390/cells12111465 [PubMed] [PMC]
Cartagena-Rivera AX, Wang WH, Geahlen RL, Raman A (2015) Fast, multi-frequency, and quantitative nanomechanical mapping of live cells using the atomic force microscope. Scientific Reports 5: 11692. https://doi.org/10.1038/srep11692[PubMed] [PMC]
Cheirdaris D (2022) Visualizing Neurodegeneration Using Atomic Force Microscopy. Handbook of Computational Neurodegeneration ( 4): 1–21. https://doi.org/10.1007/978-3-319-75479-6_4-2
Chighizola M, Dini T, Lenardi C, Milani P, Podestà A, Schulte C (2019) Mechanotransduction in neuronal cell development and functioning. Biophysical reviews 11: 701–720. https://doi.org/10.1007/s12551-019-00587-2 [PubMed][PMC]
Dabral S, Muecke C, Valasarajan C, Schmoranzer M, Wietelmann A, Semenza GL, Meister M, Muley T, Seeger-Nukpezah T, Samakovlis C, Weissmann N, Grimminger F, Seeger W, Savai R, Pullamsetti SS (2019) A RASSF1A-HIF1α loop drives Warburg effect in cancer and pulmonary hypertension. Nature Communications 10(1): 2130 https://doi.org/10.1038/s41467-019-10044-z [PubMed] [PMC]
DeGiosio RA, Grubisha MJ, MacDonald ML, McKinney BC, Camacho CJ, Sweet RA (2022) More than a marker: Potential pathogenic functions of MAP2. Frontiers in Molecular Neuroscience 15: 974890. https://doi.org/10.3389/fnmol.2022.974890 [PubMed] [PMC]
Demyanenko S, Nikul V, Rodkin S, DavletsHIn A, Evgen'ev MB, Garbuz DG (2021) Exogenous recombinant Hsp70 mediates neuroprotection after photothrombotic stroke. Cell Stress and Chaperones 26(1): 103–114. https://doi.org/10.1007/s12192-020-01159-0 [PubMed] [PMC]
Dukay B, Csoboz B, Tóth ME (2019) Heat-shock proteins in neuroinflammation. Frontiers in Pharmacology 10: 920–931 https://doi.org/10.3389/fphar.2019.00920 [PubMed] [PMC]
Frederix PL, Bosshart PD, Engel A (2009) Atomic force microscopy of biological membranes. Biophysical Journal 96(2): 329–338. http://doi.org/10.1016/j.bpj.2008.09.046 [PubMed] [PMC]
Galvanetto N (2018) Single-cell unroofing: probing topology and nanomechanics of native membranes. Biochim Biophys Acta (BBA) – Biomembranes 1860: 2532–2538. https://doi.org/10.1016/j.bbamem.2018.06.011 [PubMed]
Guimarães CF, Gasperini L, Marques AP, Reis RL (2020) The stiffness of living tissues and its implications for tissue engineering. Nature Reviews Materials 5: 351–370. https://doi.org/10.1038/s41578-019-0120-8
Guo CY, Xiong TQ, Tan BH, Gui Y, Ye N, Li SL, Li YC (2019) The temporal and spatial changes of actin cytoskeleton in the hippocampal CA1 neurons following transient global ischemia. Brain research 17(20): 1462–1497. https://doi:10.1016/j.brainres.2019.06.016 [PubMed]
Guo CY, Xiong TQ, Tan BH, Gui Y, Ye N, Li SL, Li YC (2019) The temporal and spatial changes of actin cytoskeleton in the hippocampal CA1 neurons following transient global ischemia. Brain Research 1720: 146297. https://doi.org/10.1016/j.brainres.2019.06.016 [PubMed]
Handorf AM, Zhou Y, Halanski MA, Li W-J (2015) Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 11(1): 1–15. https://doi.org/10.1080/15476278.2015.1040571 [PubMed] [PMC]
Kiio TM, Park S (2020) Nano-scientific application of atomic force microscopy in pathology: from molecules to tissues. International journal of medical sciences17: 844–858. https://doi.org/10.7150/ijms.41805 [PubMed] [PMC]
Kiral FR, Kohrs FE, Jin EJ, Hiesinger PR (2018) Rab GTPases and membrane trafficking in neurodegeneration. Current Biology 28(8): 471–486. https://doi.org/10.1016/j.cub.2018.02.010 [PubMed] [PMC]
Leu JI, Barnoud T, Zhang G, Tian T, Wei Z, Herlyn M (2017) Inhibition of stress-inducible HSP70 impairs mitochondrial proteostasis and function. Oncotarget 8: 45656–45669. https://doi.org/10.18632/oncotarget.17156 [PubMed] [PMC]
Leu JI, Barnoud T, Zhang G, Tian T, Wei Z, Herlyn M, Murphy ME, George DL (2017) Inhibition of stress-inducible HSP70 impairs mitochondrial proteostasis and function. Oncotarget 8(28): 45656–45669. https://doi.org/10.18632/oncotarget.17321 [PubMed] [PMC]
Levenson RW, Sturm VE, Haase CM (2014) Emotional and behavioral symptoms in neurodegenerative disease: a model for studying the neural bases of psychopathology. Annual review of clinical psychology 10: 581–606. https://doi.org/10.1146/annurev-clinpsy-032813-153653 [PubMed] [PMC]
Mishra AK, Tripathi MK, Kumar D, Gupta SP (2024) Neurons specialize in presynaptic autophagy: A perspective to ameliorate neurodegeneration. Molecular Neurobiology https://doi.org/10.1007/s12035-024-04399-8 [PubMed]
Nakano F, Liu L, Kawakita F, Kanamaru H, Nakatsuka Y, Nishikawa H, Okada T, Shiba M, Suzuki H (2019) Morphological characteristics of neuronal death after experimental subarachnoid hemorrhage in mice using double immunoenzymatic technique. The Journal of Histochemistry and Cytochemistry 67(12): 919–930. https://doi.org/10.1369/0022155419878181 [PubMed] [PMC]
Ohashi K, Fujiwara S, Mizuno K (2017) Roles of the cytoskeleton, cell adhesion and Rho signaling in mechanosensing and mechanotransduction. The Journal of Biochemistry 161(3): 245–254 https://doi.org/10.1038/s41578-019-0169-1[PubMed]
Papa L, Robicsek SA, Brophy GM, Wang KK, Hannay HJ, Heaton S, Schmalfuss I, Gabrielli A, Hayes RL, Robertson CS (2018) Temporal profile of microtubule-associated protein 2: A novel indicator of diffuse brain injury severity and early mortality after brain trauma. Journal of Neurotrauma 35(1):32–40. https://doi.org/10.1089/neu.2017.4994 [PubMed][PMC]
Pluta R, Ouyang L, Januszewski S, Li Y, Czuczwar SJ (2021) Participation of amyloid and tau protein in post-ischemic neurodegeneration of the hippocampus of a nature identical to Alzheimer’s disease. International Journal of Molecular Sciences 22: 2460. https://doi.org/10.3390/ijms22052460 [PubMed] [PMC]
Pluta R, Ułamek-Kozioł M, Januszewski S, Czuczwar SJ (2019) Amyloid pathology in the brain after ischemia. Folia Neuropathologica 57(3): 220–226. https://doi.org/10.5114/fn.2019.88450 [PubMed] [PubMed] [PMC]
Qin C, Yang S, Chu YH, Zhang H, Pang XW, Chen L, Zhou LQ, Chen M, Tian DS, Wang W (2022) Signaling pathways involved in ischemic stroke: molecular mechanisms and therapeutic interventions. Signal Transduction and Targeted Therapy 7: 215. https://doi.org/10.1038/s41392-022-01064-1 [PubMed] [PMC]
Radenovic L, Nenadic M, Ułamek-Kozioł M, Januszewski S, Czuczwar SJ, Andjus PR, Pluta R (2020) Heterogeneity in brain distribution of activated microglia and astrocytes in a rat ischemic model of Alzheimer’s disease after 2 years of survival. Aging 12(12): 12251–12267. https://doi.org/10.18632/aging.103411 [PubMed] [PMC]
Seano G, Nia HT, Emblem KE, Datta M, Ren J, Krishnan S, Kloepper J, Pinho MC, Ho WW, Ghosh M (2019) Solid stress in brain tumours causes neuronal loss and neurological dysfunction and can be reversed by lithium. Nature Biomedical Engineering 3: 230–245. https://doi.org/10.1038/s41551-018-0334-7 [PubMed] [PMC]
Shao A, Zhou Y, Yao Y, Zhang W, Zhang J, Deng Y (2019) The role and therapeutic potential of heat shock proteins in haemorrhagic stroke. Journal of Cellular and Molecular Medicine 23(9): 5846–5858. https://doi.org/10.1111/jcmm.14479[PubMed] [PMC]
Sheldon RA, Windsor C, Ferriero DM (2018) Strain-related differences in mouse neonatal hypoxia-ischemia. International Journal of Developmental Neuroscience 40(5-6): 490–496. https://doi.org/10.1159/000495880 [PubMed] [PMC]
Skorkina MYu, Fedorova MZ, Sladkova EA, Muravyov AV (2012) The use of nanomechanic sensor for studies of morphofunctional properties of lymphocytes from healthy donors and patients with chronic lymphoblastic leukemia. Bulletin of Experimental Biology and Medicine 154(1): 163–166. https://doi.org/10.1007/s10517-012-1899-x [PubMed]
Sun YY, Zhu HJ, Zhao RY, Zhou SY, Wang MQ, Yang Y, Guo ZN (2023) Remote ischemic conditioning attenuates oxidative stress and inflammation via the Nrf2/HO-1 pathway in MCAO mice. Redox Biology 66: 102852. https://doi.org/10.1016/j.redox.2023.102852 [PubMed] [PMC]
Tirpe AA, Gulei D, Ciortea SM, Crivii C, Berindan-Neagoe I (2019) Hypoxia: overview on hypoxia-mediated mechanisms with a focus on the role of HIF genes. International Journal of Molecular Sciences 20(24): 6140. https://doi.org/10.3390/ijms20246140 [PubMed] [PMC]
Tuo Q, Zhang S, Lei P (2021) Mechanisms of neuronal cell death in ischemic stroke and their therapeutic implications. Medicinal Research Reviews 42:259–305. https://doi.org/10.1002/med.21773 [PubMed]
Vining KH, Mooney DJ (2017) Mechanical forces direct stem cell behavior in development and regeneration. Nature Reviews Materials 18(12): 728–742. https://doi.org/10.1038/nrm.2017.81 [PubMed] [PMC]
Wilson KA, MacDermott-Opeskin HI, Riley E, Lin Y, O'Mara ML (2020) Understanding the link between lipid diversity and the biophysical properties of the neuronal plasma membrane. Biochemistry 59(33): 3010–301. https://doi.org/10.1021/acs.biochem.0c00524 [PubMed]
Wilson KA, MacDermott-Opeskin HI, Riley E, Lin Y, O'Mara ML (2020) Understanding the link between lipid diversity and the biophysical properties of the neuronal plasma membrane. Biochemistry. 59(33): 3010–3018 https://doi.org/10.1021/acs.biochem.0c00524 [PubMed]
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Pokrovsky VM, Deikin AV, T Zhang, Verlov NA, Konevega AL, Korokin MV

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

