Ascorbic acid-containing compound efficacy in ischemic brain damage
DOI:
https://doi.org/10.18413/rrpharmacology.10.508Abstract
Introduction: Ischemic brain injury remains one of the main causes of disability and mortality worldwide. Protection of cellular population, depriving from oxygen supply and nutrients, is of extreme importance for further both clinical and health outcomes of timely implemented intravascular intervention. The aim: to assess anti-ischemic activity of 3-hydroxypyridine ascorbate in the in vitro and in vivo models of brain cell and tissue response to ischemia and reoxygenation.
Materials and Methods: 3-hydroxypyridine ascorbate (laboratory code 3-EA) was assessed as chemical substance (purity 99.8%) diluted in sterile phosphate-buffered saline. Intracellular Ca2+ response to glutamate excitotoxicity (GluTox), ischemia and reoxygenation as well as cellular viability was evaluated on NMRI murine fresh cortical neuro-glial cell culture incubated with 2-EA by registering intracellular Fura-2 and propidium iodide fluorescence respectively. Expression of apoptosis regulating genes BCL-2, STAT3, SOCS3, inflammation regulating genes TRAIL, MLKL, Cas-1, Cas-3, IL-1β и TNFα, and genes MAO-A and MAO-B was determined by real-time PCR. The substance neuroprotection was studied in male Sprague-Dawley rats with intraluminal middle cerebral artery (MCA) occlusion/reperfusion treated with 18 mg/kg of 2-EA along with neurological deficiency evaluation and morphological assessment of brain sections.
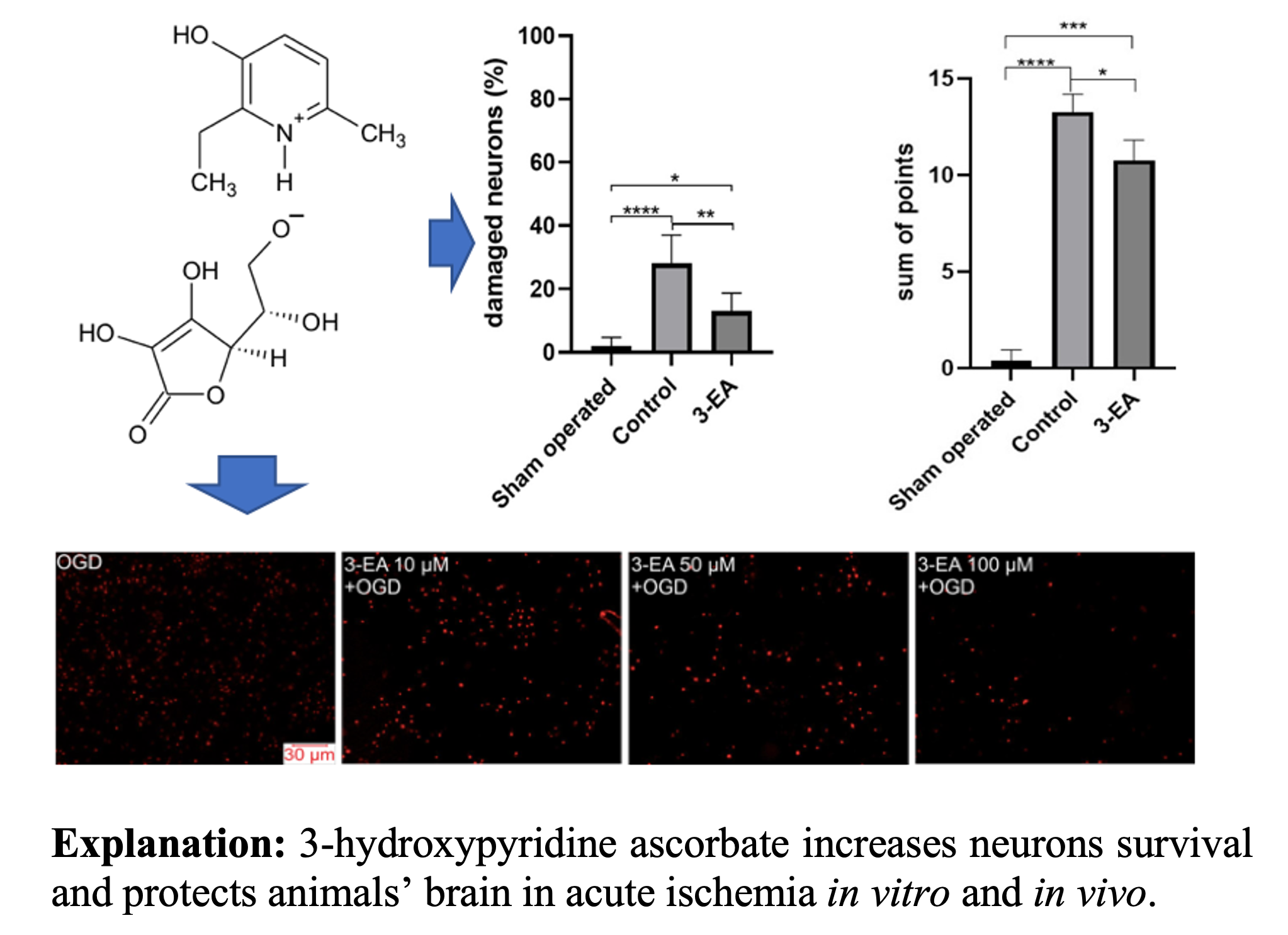
Results: Preincubation of cortical cells with 10-100 μM of 3-EA leads to inhibition of [Ca2+]i in cytosol of neurons and astrocytes under GluTox and oxygen-glucose deprivation (OGD) conditions. Reducing [Ca2+]i inhibits necrotic cell death in an acute experiment. Incubation of cerebral cortex cells with 3-EA leads to an overexpression of anti-apoptotic genes BCL-2, STAT3, SOCS3, along with downregulation of genes TRAIL, MLKL, Cas-1, Cas-3, IL-1β and TNFα. Intraperitoneal administration of 3-EA reduces the volume of necrotic areas, perinecrotic edema, cell damage, and neurological deficits in rats with MCA occlusion.
Conclusion: 3-EA dose-dependently suppresses the death of cerebral cortex cells under the excitotoxic effects of glutamate and ischemia/reoxygenation. Cell-protective effect of 3-EA involves changes in the basal and ischemia/reoxygenation-induced expression of genes encoding anti-apoptotic proteins and oxidative status proteins, which leads to inhibition of the late irreversible stages of apoptosis. A course administration of 3-hydroxypyridine ascorbate at a dose of 18 mg/kg per day reduces the severity of damage both by preserving the population of neurons in the penumbra zone and by limiting the local stress response.
Graphical Abstract
Keywords:
3-hydroxypyridine ascorbate (3-EA), ischemia, brain damage, cell culture, excitotoxicity, Ca2 , cerebral artery occlusion, neurological deficitsReferences
Bailey EL, Smith C, Sudlow CL, Wardlaw JM (2012) Pathology of lacunar ischemic stroke in humans –a systematic review. Brain Pathology22(5): 583–591.https://doi.org/10.1111/j.1750-3639.2012.00575.x [PubMed]
Balch MHH, Nimjee SM, Rink C, Hannawi Y (2020) Beyond the brain: The systemic pathophysiological response to acute ischemic stroke. Journal of Stroke 22(2): 159–172. https://doi.org/10.5853/jos.2019.02978 [PubMed]
Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H (1986) Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke 1986 17(3): 472–476. https://doi.org/10.1161/01.str.17.3.472[PubMed]
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R et al.(2017) Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation135(10): e146–e603. https://doi.org/10.1161/cir.0000000000000491 [PubMed]
Blinova E, Turovsky E, Eliseikina E, Igrunkova A, Semeleva E, Golodnev G, Termulaeva R, Vasilkina O, Skachilova S, Mazov Y, Zhandarov K, Simakina E, Belanov K, Zalogin S, Blinov D (2022). Novel hydroxypyridine compound protects brain cells against ischemic damage in vitro and in vivo. International Journal of Molecular Sciences 23(21): 12953. https://doi.org/10.3390/ijms232112953 [PubMed]
Chen YC, Ma NX, Pei ZF, Wu Z, Do-Monte FH, Keefe S, Yellin E, Chen MS, Yin JC, Lee G, Minier-Toribio A, Hu Y, Bai YT, Lee K, Quirk GJ, Chen G (2020). A neuroD1 AAV-based gene therapy for functional brain repair after ischemic injury through in vivo astrocyte-to-neuron conversion. Molecular Therapy28(1): 217–234. https://doi.org/10.1016/j.ymthe.2019.09.003 [PubMed]
GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: A systematic analysis for the Global Burden of Disease Study 2015 (2016).Lancet 388(10053): 1459–1544. https://doi.org/10.1016/s0140-6736(14)61682-2 [PubMed]
Katan M and Luft A (2018). Global burden of stroke. Seminars in Neurology 38(2): 208–211 (2018). https://doi.org/10.1055/s-0038-1649503 [PubMed]
Mirzoyan RS, Gan’shina TS, Kurdyumov IN, Maslennikov DV, Gnezdilova AV, Gorbunov AA, Kurza EV, Turilova AI, Kostochka LM, Mirzoyan NR (2021) Migraine pharmacology and brain ischemia. Research Results in Pharmacology7(2): 67–82. https://doi.org/10.3897/rrpharmacology.7.67463
Ren X, Hu H, Farooqi I, Simpkins JW (2020).Blood substitution therapy rescues the brain of mice from ischemic damage. Nature Communications11(1): 4078. https://doi.org/10.1038/s41467-020-17930-x [PubMed]
Turovsky EA, Varlamova EG, Gudkov SV, Plotnikov EY (2021) The protective mechanism of deuterated linoleic acid involves the activation of the Ca2+ signaling system of astrocytes in ischemia in vitro. International Journal of Molecular Sciences 22(24): 13216. https://doi.org/10.3390/ijms222413216 [PubMed]
Varlamova EG, Turovsky EA, Babenko EY, Plotnikov EY (2021). The mechanisms underlying the protective action of selenium nanoparticles against ischemia/reoxygenation are mediated by the activation of the Ca2+ signaling system of astrocytes and reactive astrogliosis. International Journal of Molecular Sciences 22(23): 12825. https://doi.org/10.3390/ijms222312825 [PubMed] [PubMed]
Warlow C, Sudlow C, Dennis M, Wardlaw J, Sandercock P (2003) Stroke. Lancet 362(9391): 1211–1224. https://doi.org/10.1016/S0140-6736(03)14544-8 [PubMed]
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Rita M. Termulaeva, Konstantin Y. Belanov, Natalya D. Bunyatyan, Aleksander S. Pirozhkov, Dmitrii E. Timoshkin, Ekaterina V. Blinova, Olga V. Vasilkina, Kirill D. Blinov; Elena V. Semeleva; Aleksander A. Dmitriev, Dmitrii S. Blinov

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

