Properties and antitumor activity of combined polymeric nanoparticles based on gefitinib and a photosensitizer
DOI:
https://doi.org/10.18413/rrpharmacology.11.600Abstract
Introduction: Gefitinib (GFT) is a moderately lipophilic small quinazoline molecule with proven efficacy in treating locally advanced or metastatic non-small cell lung cancer. Due to the broad activity of GFT and other tyrosine kinase inhibitors, researchers worldwide strive to create various nanoparticles based on these substances, including their combinations with other active molecules. This study investigated morphology, release, and cytotoxic activity of micellar form of combination particles with GFT and a phthalocyanine photosensitizer in various tumor models.
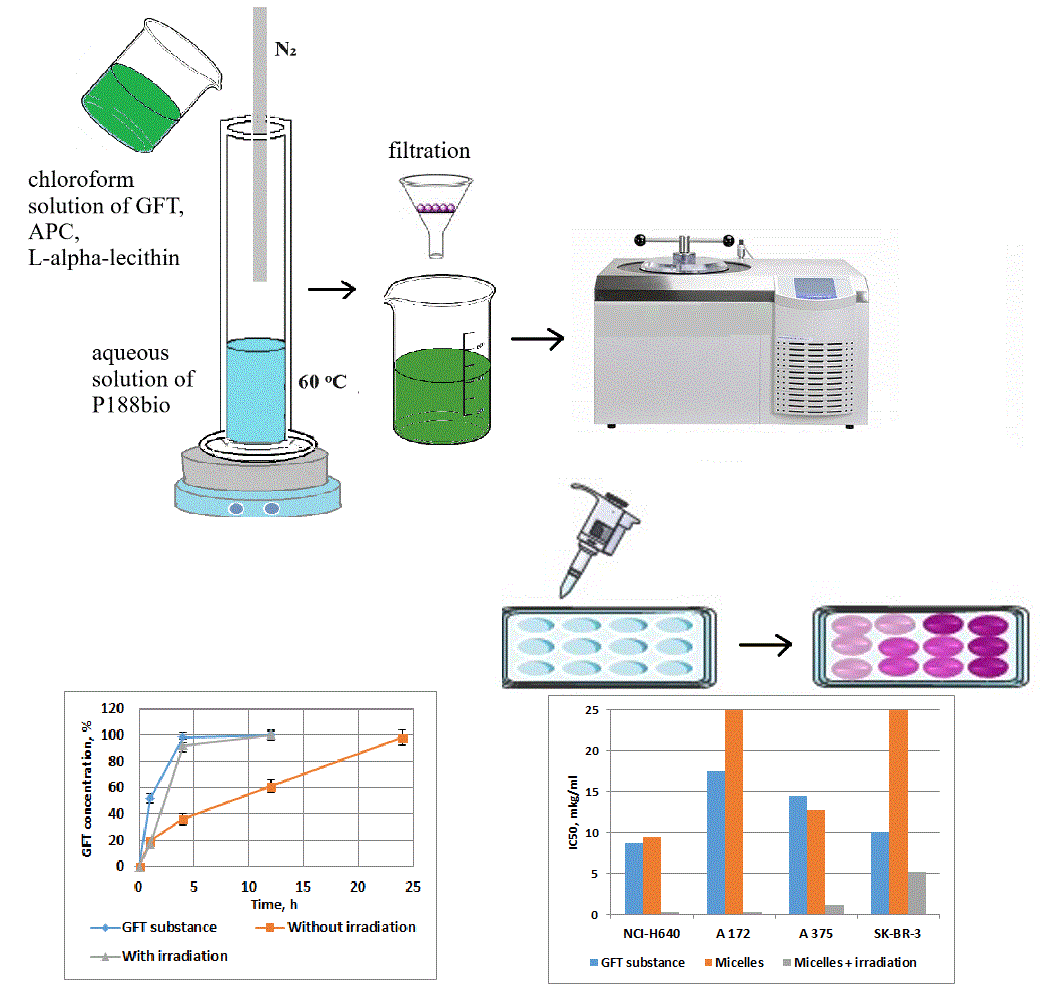
Materials and Methods: The micellar model was obtained by mixing/emulsifying the aqueous and organic phases using a continuous flow of nitrogen gas, an aqueous solution of poloxamer 188 as the aqueous phase, and a chloroform solution of the active substances as the organic phase. Conventional analytical and biological methods were used to characterize the obtained micelles. The average particle size and polydispersity index of the samples were determined by dynamic light scattering and microscopy. Release from particles was determined in vitro by quantifying free GFT released through the dialysis insert. Cytotoxic activity was studied on the cell lines of lung cancer NCI-H640, glioblastoma A 172, melanoma A 375, and breast cancer SK-BR-3.
Results and Discussion: The micelles with GFT, a photosensitizer, poloxamer 188, and polyvinylpyrrolidone were spherical and nanosized. 50% of the encapsulated GFT was released from the micellar model in a phosphate buffer medium within about 10.0 h. Testing of micelles on a panel of four tumor cell lines in the dose range of 0.1-20 μg/mL showed high cytotoxic activity of the studied model, especially for lung cancer and glioblastoma.
Conclusion: The obtained data suggest a promising in-depth study of micellar nanoparticles of GFT with a photosensitizer, especially concerning lung cancer and glioblastoma.
Graphical Abstract
Keywords:
gefitinib polymeric nanoparticles, photosensitizer, release in vitro, study of cytotoxic activity, lung cancer NCI-H640, glioblastoma A 172, melanoma A 375, breast cancer SK-BR-3References
Abo Qoura L, Karshieva SS, Borisova JA, Pokrovsky VS (2023) Cytotoxic and anticancer activity of pharmacological pairs of C115H methionine–gamma-lyase and S-propyl-L-cysteine sulfoxide. Russian Journal of Oncology 28(1): 5– https://doi.org/10.17816/onco501727
Aljuffali IA, Anwer MK, Ahmed MM, Alalaiwe A, Aldawsari MF, Fatima F, Jamil S (2023) Development of gefitinib-loaded solid lipid nanoparticles for the treatment of breast cancer: Physicochemical Evaluation, Stability, and Anticancer Activity in Breast Cancer (MCF-7) Cells. Pharmaceuticals 16(11): 1549. https://doi.org/10.3390/ph16111549
Almasoudi HH, Mashraqi MM, Alshamrani SA, Alharthi AA, Alsalmi O, Nahari MH, Al-Mansour FSH, Alhazmi AYM (2024) Structure-based in silico approaches reveal IRESSA as a multitargeted breast cancer regulatory, signalling, and receptor protein inhibitor. Pharmaceuticals 17(2): 208. https://doi.org/10.3390/ph17020208
Attili I, Corvaja C, Spitaleri G, Del Signore E, Trillo Aliaga P, Passaro A, de Marinis F (2023) New generations of tyrosine kinase inhibitors in treating NSCLC with oncogene addiction: strengths and limitations. Cancers 15(20): 5079. https://doi.org/10.3390/cancers15205079
Attili I, Karachaliou N, Conte P, Bonanno L, Rosell R (2018) Therapeutic approaches for T790M mutation positive non-small-cell lung cancer. Expert Review of Anticancer Therapy 18: 1021–1030. https://doi.org/10.1080/14737140.2018.1508347
Dickerson H, Diab A, Al Musaimi O (2024) Epidermal growth factor receptor tyrosine kinase inhibitors in cancer: current use and future prospects. International Journal of Molecular Sciences 25(18): https://doi.org/10.3390/ijms251810008
Greish K, Jasim A, Parayath N, Abdelghany S, Alkhateeb A, Taurin S, & Nehoff H (2017) Micellar formulations of Crizotinib and Dasatinib in the management of glioblastoma multiforme. Journal of Drug Targeting 26(8): 692–708. https://doi.org/10.1080/1061186X.2017.1419357
He Q, Qu M, Bao H, Xu Y, Shen T, Tan D, Barkat MQ, Xu C, Zeng LH, Wu X (2023) Multiple post-translational modifications ensure EGFR functionality: Potential therapeutic targets to overcome its drug-resistance mutations. Cytokine and Growth Factor Review 70: 41–53. https://doi.org/10.1016/j.cytogfr.2023.03.003
Ibrahim TS, Malebari AM, Mohamed MFA (2021) Design, synthesis, in vitro anticancer evaluation and molecular modelling studies of 3,4,5-trimethoxyphenyl-based derivatives as dual EGFR/HDAC hybrid inhibitors. Pharmaceuticals 14(11): 1177. https://doi.org/10.3390/ph14111177
Karami A, Hossienpour M, Mohammadi Noori E, Rahpyma M, Najafi K, Kiani A (2022) Synergistic effect of gefitinib and temozolomide on U87MG glioblastoma angiogenesis. Nutrition and Cancer 74(4): 1299– https://doi.org/10.1080/01635581.2021.1952441
Lu W, Sun J, Jing Y, Xu J, Huang C, Deng Y, Tian P, Li Y (2025) Combined use of gefitinib and bevacizumab in advanced on-small-cell lung cancer with EGFR G719S/S768I mutations and acquired C797S without T790M after osimertinib: A case report and literature review. Current Oncology 32(4): https://doi.org/10.3390/curroncol32040201
Nikolaeva LL, Sanarova EV, Kolpaksidi AP, Shcheglov SD, Rudakova AA, Baryshnikova MA, Lantsova AV (2024) Effect of the composition of combined solid lipid particles with gefitinib and a photosensitizer on their size, stability and cytotoxic activity. Biomedical Photonics 13(2): 19–25. https://doi.org/10.24931/2413-9432-2023-13-1-19-25
Sanarova EV, Lantsova AV, Nikolaeva LL, Oborotova NA, Litvinenko YaE, Solov’eva NL (2023) Creation of a model of a complex delivery nanosystem containing a tyrosine kinase inhibitor and a photosensitizer. Pharmaceutical Chemistry Journal 57: 1075– https://doi.org/10.1007/s11094-023-02986-y
Șandor A, Ionuț I, Marc G, Oniga I, Eniu D, & Oniga O (2023) Structure–activity relationship studies based on quinazoline derivatives as EGFR kinase inhibitors (2017–Present). Pharmaceuticals 16(4): 534. https://doi.org/10.3390/ph16040534
Singh R, Lillard JW Jr (2009) Nanoparticle-based targeted drug delivery. Experimental and Molecular Pathology 86(3): 215-223. https://doi.org/10.1016/j.yexmp.2008.12.004
Susova OY, Karshieva SS, Кostyukov AA, Moiseeva NI, Zaytseva EA, Kаlabina KV, Zusinaite E, Gildemann K, Smirnov NM, Аrutyunyan AF, Zhuze AL (2024) Dimeric bis-benzimidazole-pyrroles DB2Py(n) – AT-site-specific ligands: synthesis, physicochemical analysis, and biological activity. Acta Naturae 16(1): 86– https://doi.org/10.32607/actanaturae.27327
Rahman AFMM, Korashy HM, Kassem MG (2014) Gefitinib. In: Brittain HG (Ed.) Profiles of Drug Substances, Excipients and Related Methodology, Academic Press, Milford, New Jersey, 39: 239–264. https://doi.org/10.1016/B978-0-12-800173-8.00005-2
Rawluk J, Waller CF (2018) Gefitinib. In: Martens UM (Ed.) Small Molecules in Oncology. Springer International Publishing: Cham, Switzerland, 235–246.
Van den Bent MJ, Brandes AA, Rampling R, Kouwenhoven MC, Kros JM, Carpentier AF, Clement PM, Frenay M, Campone M, Baurain JF, Armand J-P, Taphoorn MJB, Kletzi H, Klughammer B, Lacombe D, Gorlia T (2009) Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. Journal of Clinical Oncology 27: 1268– https://doi.org/10.1200/JCO.2008.17.5984
Yang Z, Du Y, Lei L, Xia X, Wang X, Tong F, Li Y, Gao H (2023) Co-delivery of ibrutinib and hydroxychloroquine by albumin nanoparticles for enhanced chemotherapy of glioma. International Journal of Pharmaceutics 630: 122436. https://doi.org/10.1016/j.ijpharm.2022.122436
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Nikolaeva LL, Lantsova AV, Baryshnikova MA, Rudakova AA, Orlova OL, Bunyatyan ND, Markova AA, Kuzmin VA, Sanarova EV

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

