Anti-anxiety properties of new 5H-2,3-benzodiazepine and 5H-[1,2,5]triazepine derivatives
DOI:
https://doi.org/10.18413/rrpharmacology.11.808Abstract
Introduction: Previous studies have examined the anxiolytic properties of compounds containing mutually annulated systems of 5H-2,3-benzodiazepine and [1,2,4]triazole, namely 7H-[1,2,4]triazolo[3,4-a][2,3]benzodiazepine derivatives. In the present work, another group of 5H-2,3-benzodiazepine and 5H-[1,2,5]triazepine derivatives was examined for psychotropic activity.
Materials and Methods: For in silico assay the PASS online test system and ADMET analysis were used. Compounds under study were also tested in vivo in Elevated Plus Maze, Open Field and Rotarod tests.
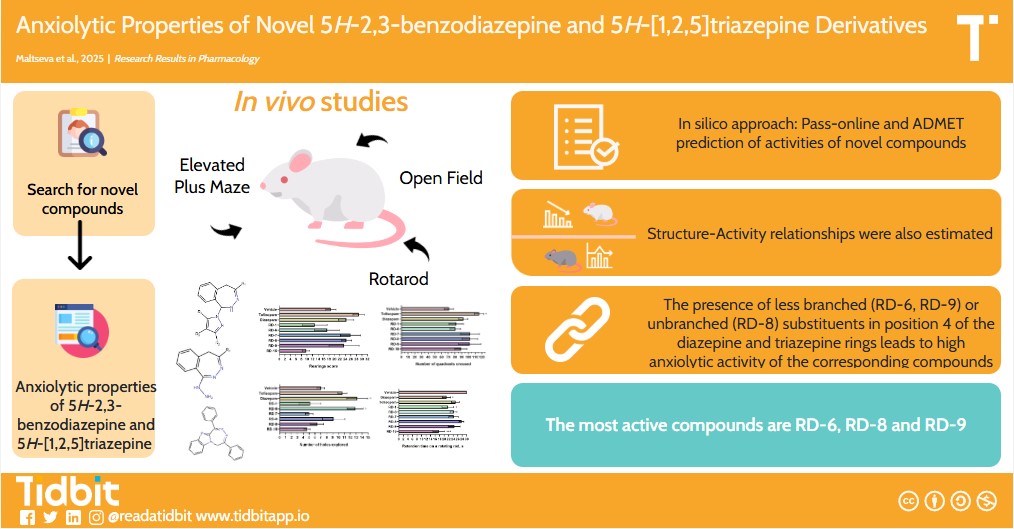
Results and Discussion: Of note is that the presence of a 3,4-dimethoxyphenyl substituent at position 4 of the diazepine ring (RD-1, RD-7) leads to a decrease in anxiolytic activity compared to 4-methoxyphenyl derivatives (RD-6, RD-9). Among the derivatives of condensed 5H-[1,2,5]triazepines, a more pronounced anti-anxiety effect was exerted by compound RD-8 with an annelated benzimidazole bicycle compared to its analog RD-15 with a pyrrole cycle. The high anxiolytic activity of the compounds is facilitated by the presence of slightly branched (RD-6, RD-9) or unbranched (RD-8) substituents in position 4 of the diazepine or triazepine cycle. In the Rotarod test, it was noted that 4-(3,4-dimethoxyphenyl)-1-hydrazino-5H-2,3-benzodiazepine (RD-1) and 4-hydrazino-7,8,9-trimethyl-1-phenyl-5H-pyrrolo[2,1-d][1,2,5]triazepine (RD-15) have a negative effect on muscle tone in mice due to the presence of a hydrazino group in their composition. According to ADMET analysis, compounds RD-6, RD-8 and RD-9 are low toxic; however, a specific toxicity study among these derivatives is recommended.
Conclusion: The most active and safe compound for further studies among the studied derivatives with a single administration is 1,4-diphenyl-5H-[1,2,5]triazepino[5,4-a]benzimidazole (RD-8) at a dose of 1.3 mg/kg.
Graphical Abstract
Keywords:
5H-2,3-benzodiazepine, 5H-[1,2,5]triazepine, ADMET, PASS online, SAR, anxiolytic, open fieldReferences
Abu-Hashem AA, Hakami O, Amri N, Mukhrish YE, Abdelgawad AAM (2024) Synthesis of 1,3,5-triazepines and benzo[f][1,3,5]triazepines and their biological activity: recent advances and new approaches. Molecules 29(3): 632. https://doi.org/10.3390/molecules29030632[PubMed] [PMC]
Alwaili MA, Abu-Almakarem AS, Aljohani S, Alkhodair SA, Al-Bazi MM, Eid TM, Alamri J, Mobasher MA, Algarzae NK, A Khayyat AI, Alshaygy LS, El-Said KS (2024) Avenanthramide-C ameliorate doxorubicin-induced hepatotoxicity via modulating Akt/GSK-3β and Wnt-4/β-Catenin pathways in male rats. Frontiers in Molecular Biosciences 11: 1507786. https://doi.org/10.3389/fmolb.2024.1507786 [PubMed] [PMC]
Amaghnouje A, Bohza S, Bohdan N, Es-Safi I, Kyrylchuk A, Achour S, El Fatemi H, Bousta D, Grafov A (2021) New 2,3-benzodiazepine derivative: synthesis, activity on central nervous system, and toxicity study in mice. Pharmaceuticals 14(8): 814. https://doi.org/10.3390/ph14080814 [PubMed] [PMC]
da Silva DM, Sanz G, Vaz BG, de Carvalho FS, Lião LM, de Oliveira DR, da Silva Moreira LK, Cardoso CS, de Brito AF, da Silva DPB, da Rocha FF, Santana IGC, Galdino PM, Costa EA, Menegatti R (2018) Tert-butyl 4-((1-phenyl-1H-pyrazol-4-yl) methyl) piperazine-1-carboxylate (LQFM104) – New piperazine derivative with antianxiety and antidepressant-like effects: Putative role of serotonergic system. Biomedicine & Pharmacotherapy 103: 546–552. https://doi.org/10.1016/j.biopha.2018.04.077 [PubMed]
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Scientific Reports 7(1): 1–13. https://doi.org/10.1038/srep42717 [PubMed] [PMC]
Dong J (2018) ADMETlab: a platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. Journal of Cheminformatics 10(1): 1–11. [PubMed] [PMC]
Groszkowski S, Wrona J (1978) Pyridazino[1,2-a]1,2,5-triazepines. Polish Journal of Pharmacology and Pharmacy 30(5): 713–715. [PubMed]
Khabarov KM, Kharaneko OI, Bogza SL (2009) 2,3-Benzodiazepine-1-thione in the synthesis of substituted and hetero-annelated 2,3-benzodiazepines. Chemistry of Heterocyclic Compounds 45: 468–474.
Kharaneko AO (2017) 7,8,9-trimethyl-1-phenyl-3H-pyrrolo[2,1-d][1,2,5]triazepin-4(5H)-one. Synthesis and reactions. Russian Journal of Organic Chemistry 53(5): 738–745. https://doi.org/10.1134/S1070428017050153
Kharaneko AO (2019) 1,4-Diphenyl-5H-[1,2,5]triazepino[5,4-a]benzimidazole – a new heterocyclic system. Synthesis and Properties. Russian Journal of Organic Chemistry 55(1): 115–117. https://doi.org/0.1134/S1070428019010147
Kraeuter AK, Guest PC, Sarnyai Z (2019) The elevated plus maze test for measuring anxiety-like behavior in rodents. Methods in Molecular Biology 1916: 69–74. https://doi.org/10.1007/978-1-4939-8994-2_4 [PubMed]
Lubrich C, Giesler P, Kipp M (2022) Motor behavioral deficits in the cuprizone model: validity of the rotarod test paradigm. International Journal of Molecular Sciences 23(19): 11342. [PubMed] [PMC]
Nawrocka W, Liszkiewicz H, Wilimowski M, Rutkowska M, Barczyńska J, Kedzierska-Goździk L, Wojewódzki W, Szelag A (1994) Carboxylic esters derivatives of 2-thioxo-1H-2,3,4,5-tetrahydropyrido-[2,3-e]-1,3,4-triazepin-5-ones and 2-thioxo-1H-2,3,4,5-tetrahydro-1,3,4-benzotriazepin-5-ones. Acta Poloniae Pharmaceutica 51(3): 243–248. [PubMed]
Pires DEV, Blundell TL, Ascher DB (2015) pkCSM: predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. Journal of Medicinal Chemistry 58(9): 4066–4072. [PubMed] [PMC]
Rudik AV, Dmitriev AV, Lagunin AA, Filimonov DA, Poroikov VV (2019) PASS-based prediction of metabolites detection in biological systems. SAR and QSAR in Environmental Research 30(10): 751–758. https://doi.org/10.1080/1062936X.2019.1665099 [PubMed]
Skripka MO, Spasov AA, Maltsev DV (2021) Screening of anxiolytic properties and analysis of structure-activity relationship of new derivatives of 6-(4-methoxy)-7H-[1,2,4]triazolo[3,4-a][2,3]benzodiazepine under the code RD. Research Results in Pharmacology 7(2): 31–37. https://doi.org/10.3897/rrpharmacology.7.67499
Stefancich G, Massa S, Artico M (1991) Polycyclic diazepine and triazepine derivatives of pharmacological interest. Farmaco 46(1 Suppl): 297–306. [PubMed]
Üçel Uİ, Can ÖD, Demir Özkay Ü, Ulupinar E (2020) Antiamnesic effects of tofisopam against scopolamine-induced cognitive impairments in rats. Pharmacology, Biochemistry, and Behavior 190: 172858. https://doi.org/10.1016/j.pbb.2020.172858 [PubMed]
Yang H, Lou C, Sun L, Li J, Cai Y, Wang Z, Li W, Liu G, Tang Y (2019) AdmetSAR 2.0: web-service for prediction and optimization of chemical ADMET properties. Bioinformatics (Oxford, England) 35(6): 1067–1069. https://doi.org/10.1093/bioinformatics/bty707 [PubMed]
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Maltseva MO, Maltsev DV, Miroshnikov MV, Divaeva LN, Kharaneko AO, Kharaneko OI, Morkovnik AS, Spasov AA

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

