The prefrontal cortex as a target for the atypical antipsychotic RU-31 with 5-HT2A antagonistic activity in the treatment of cognitive symptoms
DOI:
https://doi.org/10.18413/rrpharmacology.11.537Abstract
Introduction: Cognitive deficits in schizophrenia are poorly managed by current antipsychotics, making the 5-HT2A receptor a promising therapeutic target, given its influence on dopaminergic signaling in the prefrontal cortex (PFC) and its involvement in cognitive processes. RU-31 (1-(2-diethylaminoethyl)-2-(4-methoxyphenyl)-imidazo[1,2-a]benzimidazole), a selective 5-HT2A antagonist, may offer cognitive benefits without the side effects associated with traditional dopamine-targeted therapies.
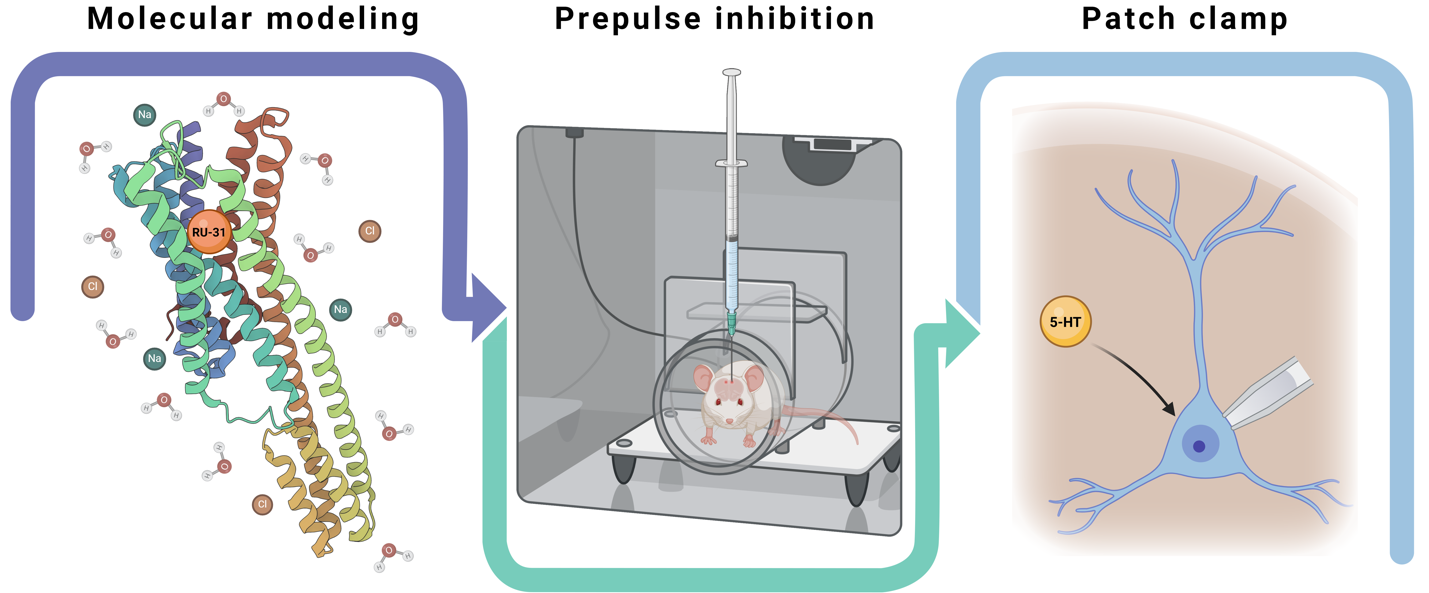
Materials and Methods: We evaluated RU-31 in Sprague-Dawley rats using molecular modeling, in vivo behavioral testing, and ex vivo electrophysiology. Molecular docking and dynamics simulations assessed the binding properties of RU-31 to the 5-HT2A receptor. The prepulse inhibition (PPI) paradigm (n=40) was used to test sensorimotor gating following a single RU-31 microinjection into the PFC. Patch-clamp recordings (n=32) from PFC pyramidal neurons were used to investigate RU-31's effects on serotonergic signaling.
Results: Molecular modeling indicated a strong binding affinity of RU-31 to the 5-HT2A receptor (100 ns). RU-31 (30 μg) restored PPI levels by 34.94% (p<0.05) following ketamine-induced deficits, suggesting an improvement in sensorimotor gating. In patch-clamp recordings, RU-31 (10 μM) significantly reduced 5-HT-mediated outward currents in layer 6 pyramidal neurons by 59.11% to 20.54 pA (SEM=7.12; SD=20.14; p<0.0211), indicating potent 5-HT2A antagonism and potential enhancement of downstream signaling.
Conclusion: This study establishes RU-31 as a promising therapeutic agent for the cognitive symptoms of schizophrenia, demonstrating that it reverses deficits in sensorimotor gating and normalizes PFC neuronal activity through selective 5-HT2A antagonism.
Graphical Abstract
Keywords:
atypical antipsychotics, 5-HT2A receptors, benzimidazoles, molecular docking, molecular dynamics, patch clamp, prepulse inhibition, prefrontal cortex, schizophrenia, cognitive deficitsReferences
Bortolozzi A, Masana M, Díaz-Mataix L, Cortés R, Scorza MC, Gingrich JA, Toth M, Artigas F (2010) Dopamine release induced by atypical antipsychotics in prefrontal cortex requires 5-HT(1A) receptors but not 5-HT(2A) receptors. The International Journal of Neuropsychopharmacology 13(10): 1299–1314. https://doi.org/10.1017/S146114571000009X[PubMed] [PMC]
Bubser M, Koch M (1994) Prepulse inhibition of the acoustic startle response of rats is reduced by 6-hydroxydopamine lesions of the medial prefrontal cortex. Psychopharmacology 113(3-4): 487–492. https://doi.org/10.1007/BF02245228 [PubMed]
Celada P, Puig MV, Artigas F (2013) Serotonin modulation of cortical neurons and networks. Frontiers in Integrative Neuroscience 7: 25. https://doi.org/10.3389/fnint.2013.00025 [PubMed] [PMC]
De Deurwaerdère P, Chagraoui A, Di Giovanni G (2021) Serotonin/dopamine interaction: Electrophysiological and neurochemical evidence. Progress in Brain Research 261: 161–264. https://doi.org/10.1016/bs.pbr.2021.02.001 [PubMed]
Frohlich J, Van Horn JD (2014) Reviewing the ketamine model for schizophrenia. Journal of Psychopharmacology (Oxford, England) 28(4): 287–302. https://doi.org/10.1177/0269881113512909
Gebreegziabhere Y, Habatmu K, Mihretu A, Cella M, Alem A (2022) Cognitive impairment in people with schizophrenia: an umbrella review. European Archives of Psychiatry and Clinical Neuroscience 272(7): 1139–1155. https://doi.org/10.1007/s00406-022-01416-6 [PubMed] [PMC]
Habtewold TD, Rodijk LH, Liemburg EJ, Sidorenkov G, Boezen HM, Bruggeman R, Alizadeh B Z (2020) A systematic review and narrative synthesis of data-driven studies in schizophrenia symptoms and cognitive deficits. Translational Psychiatry 10(1): 244. https://doi.org/10.1038/s41398-020-00919-x [PubMed] [PMC]
Hanson KL, Grant SE, Funk LH, Schumann CM, Bauman MD (2022) Impact of maternal immune activation on nonhuman primate prefrontal cortex development: insights for schizophrenia. Biological Psychiatry 92(6): 460–469. https://doi.org/10.1016/j.biopsych.2022.04.004 [PubMed] [PMC]
Howes OD, Bukala BR, Beck K (2024) Schizophrenia: from neurochemistry to circuits, symptoms and treatments. Nature Reviews. Neurology 20(1): 22–35. https://doi.org/10.1038/s41582-023-00904-0 [PubMed]
Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O’Laughlin IA, Meltzer HY (2001) 5-HT(2A) and D(2) receptor blockade increases cortical DA release via 5-HT(1A) receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. Journal of Neurochemistry 76(5): 1521–1531. https://doi.org/10.1046/j.1471-4159.2001.00154.x[PubMed]
Kalitin KY, Pridvorov GV, Spasov AA, Mukha OY (2022) Effect of clozapine and 5-HT2A-antagonist RU-31 on electroencephalography and motor activity of rats in a model of schizophrenia with neonatal destruction of the ventral hippocampus. Kuban Scientific Medical Bulletin 29(5): 108-122. https://doi.org/10.25207/1608-6228-2022-29-5-108-122
Lobo MC, Whitehurst TS, Kaar SJ, Howes OD (2022) New and emerging treatments for schizophrenia: a narrative review of their pharmacology, efficacy and side effect profile relative to established antipsychotics. Neuroscience and Biobehavioral Reviews 132: 324–361. https://doi.org/10.1016/j.neubiorev.2021.11.032 [PubMed]
López-Gil X, Babot Z, Amargós-Bosch M, Suñol C, Artigas F, Adell A (2007) Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 32(10): 2087–2097. https://doi.org/10.1038/sj.npp.1301356 [PubMed]
McCutcheon RA, Keefe RS.E, McGuire PK (2023) Cognitive impairment in schizophrenia: aetiology, pathophysiology, and treatment. Molecular Psychiatry 28(5): 1902–1918. https://doi.org/10.1038/s41380-023-01949-9 [PubMed] [PMC]
McLean SL, Harte MK, Neill JC, Young AM (2017) Dopamine dysregulation in the prefrontal cortex relates to cognitive deficits in the sub-chronic PCP-model for schizophrenia: A preliminary investigation. Journal of Psychopharmacology (Oxford, England) 31(6): 660–666. https://doi.org/10.1177/0269881117704988 [PubMed]
Menon, V, D'Esposito M (2022) The role of PFC networks in cognitive control and executive function. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 47(1): 90–103. https://doi.org/10.1038/s41386-021-01152-w[PubMed] [PMC]
Mocci G, Jiménez-Sánchez L, Adell A, Cortés R, Artigas F (2014). Expression of 5-HT2A receptors in prefrontal cortex pyramidal neurons projecting to nucleus accumbens. Potential relevance for atypical antipsychotic action. Neuropharmacology 79: 49–58. https://doi.org/10.1016/j.neuropharm.2013.10.021 [PubMed]
Nikolaus S, Chao OY, Henke J, Beu M, Fazari B, Almeida FR, Abdel-Hafiz L, Antke C, Hautzel H, Mamlins E, Müller HW, Huston JP, von Gall C, Giesel FL (2024) 5-HT1A and 5-HT2A receptor effects on recognition memory, motor/exploratory behaviors, emotionality and regional dopamine transporter binding in the rat. Behavioural Brain Research 469: 115051. https://doi.org/10.1016/j.bbr.2024.115051 [PubMed]
Pelevin A, Kurzina N, Zavialov V, Volnova A (2023) A custom solution for acoustic startle response setup with spike2-based data acquisition interface. Methods and Protocols 6(3): 57. https://doi.org/10.3390/mps6030057 [PubMed] [PMC]
Singh S, Khanna D, Kalra S (2020) Role of neurochemicals in schizophrenia. Current Psychopharmacology 9(2): 144-161. https://doi.org/10.2174/2211556009666200401150756
Swerdlow NR, Light GA (2016) Animal models of deficient sensorimotor gating in schizophrenia: are they still relevant?. Current Topics in Behavioral Neurosciences 28: 305–325. https://doi.org/10.1007/7854_2015_5012 [PubMed]
Tian MK, Schmidt EF, Lambe EK (2016) Serotonergic suppression of mouse prefrontal circuits implicated in task attention. eNeuro 3(5): ENEURO.0269-16.2016. https://doi.org/10.1523/ENEURO.0269-16.2016 [PubMed] [PMC]
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Kalitin KY, Mukha OY, Voynov VB, Spasov AA

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

