Aversion-related effects of kappa-opioid agonist U-50488 on neural activity and functional connectivity between amygdala, ventral tegmental area, prefrontal cortex, hippocampus, and nucleus accumbens
DOI:
https://doi.org/10.18413/rrpharmacology.9.10051Abstract
Introduction: Among the various receptor systems in the brain, the opioid receptors have been the subject of extensive research due to their integral role in pain modulation, reward processing, and emotional regulation. The kappa-opioid receptor (KOR) system, in particular, stands apart due to its unique contribution to stress response, aversive behaviors, and dysphoric states. This paper aims to provide an understanding of the neural activity underlying the aversion-associated effects of the KOR agonist U-50488.
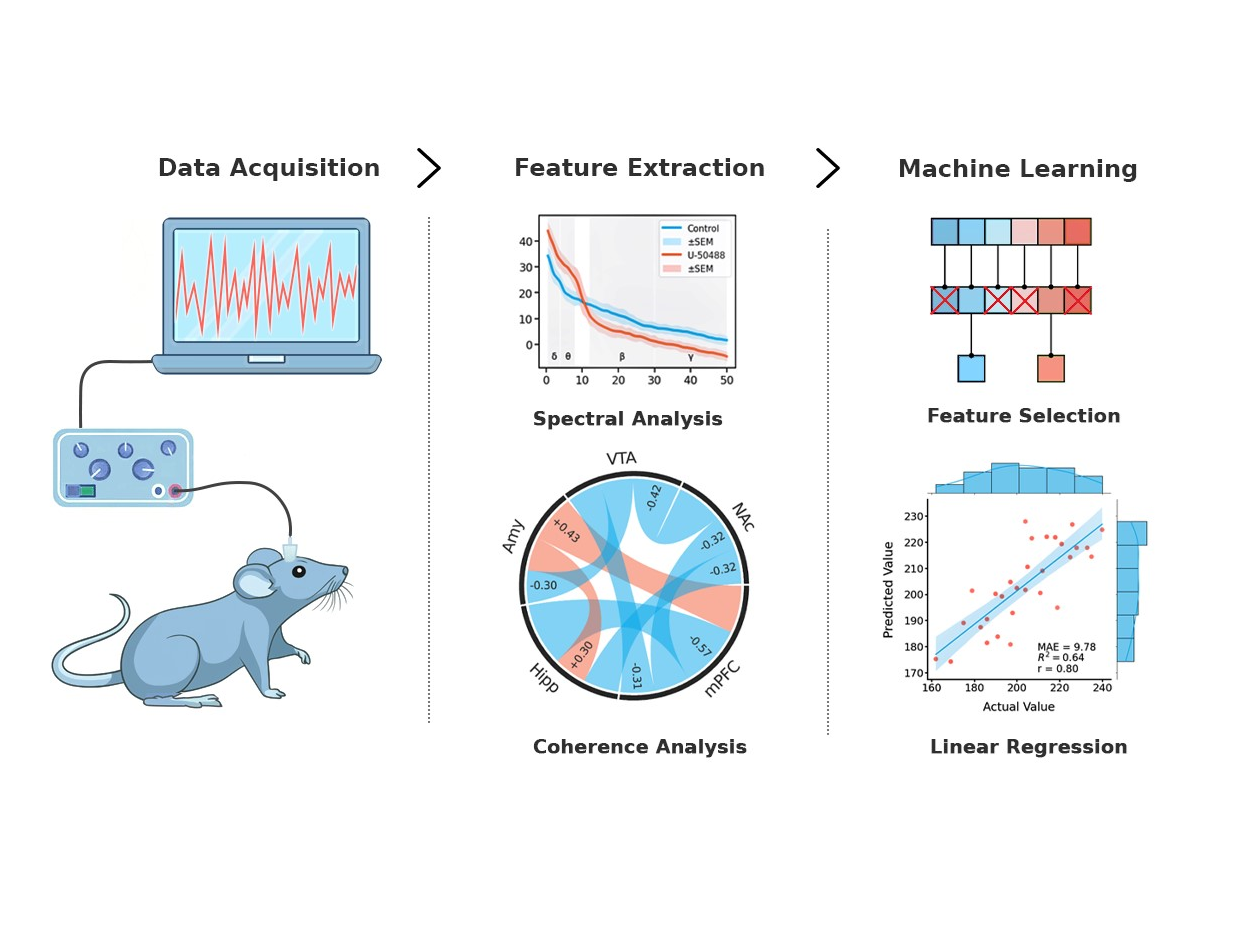
Materials and Methods: Rats underwent stereotaxic surgery to implant electrodes into the amygdala, ventral tegmental area, prefrontal cortex, hippocampus, and nucleus accumbens. The rats were subjected to conditioned place preference test to measure aversion to U-50488. After that, local field potential (LFP) recordings were made. LFP data were processed and analyzed using spectral and coherence analysis methods. A stepwise multiple linear regression was employed to identify the LFP features most significantly correlated with aversion to U-50488.
Results: The administration of U-50488 resulted in significant changes in LFP signals across multiple brain regions. These changes were particularly notable in the theta, gamma, and delta bands of brain waves (p<0.05). Theta and gamma activities were especially sensitive to the effects of U-50488. Connectivity calculations revealed shifts in coherence between brain regions, particularly highlighting the amygdala's involvement. While changes were also observed in the ventral tegmental area, prefrontal cortex, hippocampus, and nucleus accumbens (p<0.05), they contributed less to aversion. Using the stepwise multiple linear regression method, we established a final model with the 3 most significant variables: (1) coherence between the amygdala and medial prefrontal cortex, (2) coherence between the amygdala and hippocampus, and (3) theta power in the amygdala.
Conclusion: Overall, the data provided insights into how electrical neural activity mediates aversion in response to KOR activation. The results showed that the severity of aversion can be reasonably predicted (r = 0.72±0.02, p = 0.0099) using LFP band power and functional connectivity data. We concluded that the amygdala is a brain region that contributes the most to the KOR agonist-induced aversion.
Graphical Abstract
Keywords:
Selective kappa-opioid agonist, U-50488, aversion, dysphoria, amygdala, LFP, analysis, coherence, machine learningReferences
Bambico FR, Nguyen NT, Gobbi G (2009) Decline in serotonergic firing activity and desensitization of 5-HT1A autoreceptors after chronic unpredictable stress. European Neuropsychopharmacology 19(3): 215–228. https://doi.org/10.1016/j.euroneuro.2008.11.005[PubMed]
Bressler SL (2021) Directed Interregional Brain Interactions. In: Diwadkar VA, B Eickhoff S (Eds) Brain Network Dysfunction in Neuropsychiatric Illness. Springer, Cham, 75–92. https://doi.org/10.1007/978-3-030-59797-9_4
Coleman BC, Manz KM, Grueter BA (2021) Kappa opioid receptor modulation of excitatory drive onto nucleus accumbens fast-spiking interneurons. Neuropsychopharmacology 46(13): 2340–2349. https://doi.org/10.1038/s41386-021-01146-8 [PubMed] [PMC]
Coltro Campi C, Clarke GD (1995) Effects of highly selective kappa-opioid agonists on EEG power spectra and behavioural correlates in conscious rats. Pharmacology, Biochemistry, and Behavior 51(4): 611–616. https://doi.org/10.1016/0091-3057(94)00384-u [PubMed]
Corral-Frias NS, Lahood RP, Edelman-Vogelsang KE, French ED, Fellous JM (2013) Involvement of the ventral tegmental area in a rodent model of post-traumatic stress disorder. Neuropsychopharmacology 38(2): 350–363. https://doi.org/10.1038/npp.2012.189[PubMed] [PMC]
Crofton EJ, Nenov MN, Zhang Y, Scala F, Page SA, McCue DL, Li D, Hommel JD, Laezza F, Green TA (2017) Glycogen synthase kinase 3 beta alters anxiety-, depression-, and addiction-related behaviors and neuronal activity in the nucleus accumbens shell. Neuropharmacology 117: 49–60. https://doi.org/10.1016/j.neuropharm.2017.01.020 [PubMed] [PMC]
Czéh B, Vardya I, Varga Z, Febbraro F, Csabai D, Martis LS, Højgaard K, Henningsen K, Bouzinova EV, Miseta A, Jensen K, Wiborg O (2018) Long-term stress disrupts the structural and functional integrity of gabaergic neuronal networks in the medial prefrontal cortex of rats. Frontiers in Cellular Neuroscience 12: 148. https://doi.org/10.3389/fncel.2018.00148 [PubMed] [PMC]
Friedman A, Lax E, Abraham L, Tischler H, Yadid G (2012) Abnormality of VTA local field potential in an animal model of depression was restored by patterned DBS treatment. European Neuropsychopharmacology 22(1): 64–71. https://doi.org/10.1016/j.euroneuro.2011.04.005 [PubMed]
Guan P, Huang C, Lan Q, Huang S, Zhou P, Zhang C (2022) Activation of ventral tegmental area dopaminergic neurons ameliorates anxiety-like behaviors in single prolonged stress-induced PTSD model rats. Neurochemistry International 161: 105424. https://doi.org/10.1016/j.neuint.2022.105424 [PubMed]
Guidera JA, Taylor NE, Lee JT, Vlasov KY, Pei J, Stephen EP, Mayo JP, Brown EN, Solt K (2017) Sevoflurane induces coherent slow-delta oscillations in rats. Frontiers in Neural Circuits 11: 36. https://doi.org/10.3389/fncir.2017.00036 [PubMed] [PMC]
Jia L, Sun Z, Shi D, Wang M, Jia J, He Y, Xue F, Ren Y, Yang J, Ma X (2019) Effects of different patterns of electric stimulation of the ventromedial prefrontal cortex on hippocampal-prefrontal coherence in a rat model of depression. Behavioural Brain Research 356: 179–188. https://doi.org/10.1016/j.bbr.2018.08.032 [PubMed]
Kabbara A, Robert G, Khalil M, Verin M, Benquet P, Hassan M (2022) An electroencephalography connectome predictive model of major depressive disorder severity. Scientific Reports 12(1): 6816. https://doi.org/10.1038/s41598-022-10949-8 [PubMed] [PMC]
Kalitin KY, Grechko OY, Spasov AA, Sukhov AG, Anisimova VA, Matukhno AE (2018) GABAergic mechanism of anticonvulsive effect of chemical agent RU-1205. Bulletin of Experimental Biology and Medicine 164(5): 629–635. https://doi.org/10.1007/s10517-018-4047-4 [PubMed]
Kalitin KY, Pridvorov GV, Spasov AA, Mukha OY (2022a) Effect of clozapine and 5-nt2a-antagonist ru-31 on electroencephalography and motor activity of rats in a model of schizophrenia with neonatal destruction of the ventral hippocampus. Kuban Scientific Medical Bulletin [Kubanskiy Nauchnyy Meditsinskiy Vestnik] 29(5): 108–122. https://doi.org/10.25207/1608-6228-2022-29-5-108-122 [in Russian]
Kalitin KY, Pridvorov GV, Spasov AA, Mukha OY (2022b) Neuroprotective action of butorphanol and RU-1205 with evaluation of their effect on the bioelectrical activity of the brain in the global ischemia model. Journal of Volgograd State Medical University [Vestnik Volgogradskogo Gosudarstvennogo Meditsinskogo Universiteta] 19(3): 128–133. https://doi.org/10.19163/1994-9480-2022-19-3-128-133 [in Russian]
Kawashima I, Kumano H (2017) Prediction of mind-wandering with electroencephalogram and non-linear regression modeling. Frontiers in Human Neuroscience 11: 365. https://doi.org/10.3389/fnhum.2017.00365 [PubMed] [PMC]
Khan MIH, Sawyer BJ, Akins NS, Le HV (2022) A systematic review on the kappa opioid receptor and its ligands: New directions for the treatment of pain, anxiety, depression, and drug abuse. European Journal of Medicinal Chemistry 243: 114785. https://doi.org/10.1016/j.ejmech.2022.114785 [PubMed]
Lee RS, Steffensen SC, Henriksen SJ (2001) Discharge profiles of ventral tegmental area GABA neurons during movement, anesthesia, and the sleep-wake cycle. The Journal of Neuroscience 21(5): 1757–1766. https://doi.org/10.1523/jneurosci.21-05-01757.2001 [PubMed] [PMC]
Liu R, Wang Y, Chen X, Zhang Z, Xiao L, Zhou Y (2021) Anhedonia correlates with functional connectivity of the nucleus accumbens subregions in patients with major depressive disorder. NeuroImage: Clinical 30: 102599. https://doi.org/10.1016/j.nicl.2021.102599[PubMed] [PMC]
Lowes DC, Chamberlin LA, Kretsge LN, Holt ES, Abbas AI, Park AJ, Yusufova L, Bretton ZH, Firdous A, Enikolopov AG, Gordon JA, Harris AZ (2021) Ventral tegmental area GABA neurons mediate stress-induced blunted reward-seeking in mice. Nature Communications 12(1): 3539. https://doi.org/10.1038/s41467-021-23906-2 [PubMed] [PMC]
Lv X, Zhang X, Zhao Q, Li C, Zhang T, Yang X (2022) Acute stress promotes brain oscillations and hippocampal-cortical dialog in emotional processing. Biochemical and Biophysical Research Communications 598: 55–61. https://doi.org/10.1016/j.bbrc.2022.01.116[PubMed]
Markovic T, Pedersen CE, Massaly N, Vachez YM, Ruyle B, Murphy CA, Abiraman K, Shin JH, Garcia JJ, Yoon HJ, Alvarez VA, Bruchas MR, Creed MC, Morón JA (2021) Pain induces adaptations in ventral tegmental area dopamine neurons to drive anhedonia-like behavior. Nature Neuroscience 24(11): 1601–1613. https://doi.org/10.1038/s41593-021-00924-3 [PubMed] [PMC]
Merino E, Raya-Salom D, Teruel-Martí V, Adell A, Cervera-Ferri A, Martínez-Ricós J (2021) Effects of acute stress on the oscillatory activity of the hippocampus-amygdala-prefrontal cortex network. Neuroscience 476: 72–89. https://doi.org/10.1016/j.neuroscience.2021.09.009 [PubMed]
Neves GA, Grace AA (2019) α7 nicotinic receptor full agonist reverse basolateral amygdala hyperactivity and attenuation of dopaminergic neuron activity in rats exposed to chronic mild stress. European Neuropsychopharmacology 29(12): 1343–1353. https://doi.org/10.1016/j.euroneuro.2019.09.009 [PubMed] [PMC]
Okonogi T, Sasaki T (2021) Theta-range oscillations in stress-induced mental disorders as an oscillotherapeutic target. Frontiers in Behavioral Neuroscience 15: 698753. https://doi.org/10.3389/fnbeh.2021.698753 [PubMed] [PMC]
Ona G, Sampedro F, Romero S, Valle M, Camacho V, Migliorelli C, Mañanas MÁ, Antonijoan RM, Puntes M, Coimbra J, Ballester MR, Garrido M, Riba J (2022) The Kappa opioid receptor and the sleep of reason: Cortico-subcortical imbalance following salvinorin-A. The International Journal of Neuropsychopharmacology 25(1): 54–63. https://doi.org/10.1093/ijnp/pyab063 [PubMed] [PMC]
O'Neill PK, Gore F, Salzman CD (2018) Basolateral amygdala circuitry in positive and negative valence. Current Opinion in Neurobiology 49: 175–183. https://doi.org/10.1016/j.conb.2018.02.012 [PubMed] [PMC]
Page S, Mavrikaki MM, Lintz T, Puttick D, Roberts E, Rosen H, Carroll FI, Carlezon WA, Chartoff EH (2019) Behavioral pharmacology of novel kappa opioid receptor antagonists in rats. The International Journal of Neuropsychopharmacology 22(11): 735–745.https://doi.org/10.1093/ijnp/pyz054 [PubMed] [PMC]
Ranganathan M, Schnakenberg A, Skosnik PD, Cohen BM, Pittman B, Sewell RA, D’Souza DC (2012) Dose-related behavioral, subjective, endocrine, and psychophysiological effects of the κ opioid agonist Salvinorin A in humans. Biological Psychiatry 72(10): 871–879. https://doi.org/10.1016/j.biopsych.2012.06.012 [PubMed] [PMC]
Ren Z, Daker RJ, Shi L, Sun J, Beaty RE, Wu X, Chen Q, Yang W, Lyons IM, Green AE, Qiu J (2021) Connectome-based predictive modeling of creativity anxiety. Neuroimage 225: 117469. https://doi.org/10.1016/j.neuroimage.2020.117469 [PubMed]
Reznikov R, Bambico FR, Diwan M, Raymond RJ, Nashed MG, Nobrega JN, Hamani C (2018) Prefrontal cortex deep brain stimulation improves fear and anxiety-like behavior and reduces basolateral amygdala activity in a preclinical model of posttraumatic stress disorder. Neuropsychopharmacology 43(5): 1099–1106. https://doi.org/10.1038/npp.2017.207 [PubMed] [PMC]
Salin A, Dugast E, Lardeux V, Solinas M, Belujon P (2023) The amygdala-ventral pallidum pathway contributes to a hypodopaminergic state in the ventral tegmental area during protracted abstinence from chronic cocaine. British Journal of Pharmacology 180(14): 1819–1831. https://doi.org/10.1111/bph.16034 [PubMed]
Sang K, Bao C, Xin Y, Hu S, Gao X, Wang Y, Bodner M, Zhou YD, Dong XW (2018) Plastic change of prefrontal cortex mediates anxiety-like behaviors associated with chronic pain in neuropathic rats. Molecular Pain 14: 1744806918783931. https://doi.org/10.1177/1744806918783931 [PubMed] [PMC]
Simón-Arceo K, González-Trujano ME, Coffeen U, Fernández-Mas R, Mercado F, Almanza A, Contreras B, Jaimes O, Pellicer F (2017) Neuropathic and inflammatory antinociceptive effects and electrocortical changes produced by Salvia divinorum in rats. Journal of Ethnopharmacology 206: 115–124. https://doi.org/10.1016/j.jep.2017.05.016 [PubMed]
Slawecki CJ (2002) Altered EEG responses to ethanol in adult rats exposed to ethanol during adolescence. Alcoholism, Clinical and Experimental Research 26(2): 246–254. [PubMed]
Soltesz I, Deschênes M (1993) Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketamine-xylazine anesthesia. Journal of Neurophysiology 70(1): 97–116. https://doi.org/10.1152/jn.1993.70.1.97 [PubMed]
Stujenske JM, Likhtik E, Topiwala MA, Gordon JA (2014) Fear and safety engage competing patterns of theta-gamma coupling in the basolateral amygdala. Neuron 83(4): 919–933. https://doi.org/10.1016/j.neuron.2014.07.026 [PubMed] [PMC]
Sysoev YI, Prikhodko VA, Idiyatullin RD, Chernyakov RT, Karev VE, Okovityi SV (2022a) A method for chronic registration of brain cortical electrical activity in rats. Journal of Evolutionary Biochemistry and Physiology 58(1): 292–301. https://doi.org/10.1134/S0022093022010252
Sysoev YI, Prikhodko VA, Shits DD, Puchik MM, Okovityi SV (2022b) Application of the method of pharmacoencephalography for the assessment of neuroprotective drug activity. Bulletin of Perm University. Biology 4: 347–351. https://doi.org/10.17072/1994-9952-2022-4-347-351
Tortella FC, Rose J, Robles L, Moreton JE, Hughes J, Hunter JC (1997) EEG spectral analysis of the neuroprotective kappa opioids enadoline and PD117302. The Journal of Pharmacology and Experimental Therapeutics 282(1): 286–293. [PubMed]
Voget M, Rummel J, Avchalumov Y, Sohr R, Haumesser JK, Rea E, Mathé AA, Hadar R, van Riesen C, Winter C (2015) Altered local field potential activity and serotonergic neurotransmission are further characteristics of the Flinders sensitive line rat model of depression. Behavioural Brain Research 291: 299–305. https://doi.org/10.1016/j.bbr.2015.05.027 [PubMed]
Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iñiguez SD, Cao JL, Kirk A, Chakravarty S, Kumar A, Krishnan V, Neve RL, Cooper DC, Bolaños CA, Barrot M, McClung CA, Nestler EJ (2009) CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nature Neuroscience 12(2): 200–209. https://doi.org/10.1038/nn.2257 [PubMed] [PMC]
Wang L, Shen M, Jiang C, Ma L, Wang F (2016) Parvalbumin interneurons of central amygdala regulate the negative affective states and the expression of corticotrophin-releasing hormone during morphine withdrawal. The International Journal of Neuropsychopharmacology 19(11): 1–12. https://doi.org/10.1093/ijnp/pyw060 [PubMed] [PMC]
Wang W, Sun D, Pan B, Roberts CJ, Sun X, Hillard CJ, Liu QS (2010) Deficiency in endocannabinoid signaling in the nucleus accumbens induced by chronic unpredictable stress. Neuropsychopharmacology 35(11): 2249–2261. https://doi.org/10.1038/npp.2010.99 [PubMed] [PMC]
Young GA, Hudson GM, Stamidis H, Steinfels GF (1993) Interactions between U-50,488H and sigma receptor antagonists: EEG, EEG power spectral and behavioral correlates. European Journal of Pharmacology 231(3): 473–476. https://doi.org/10.1016/0014-2999(93)90127-4 [PubMed]
Zhao S, Xu X, Xie G, Zhang T (2022) Chronic corticosterone exposure impairs emotional regulation and cognitive function through disturbing neural oscillations in mice. Behavioural Brain Research 434: 114030. https://doi.org/10.1016/j.bbr.2022.114030 [PubMed]
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Константин Ю. Калитин, Александр А. Спасов, Ольга Ю. Муха Mukha

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

