Current concepts of pathogenesis of depressive disorder: A literature review
DOI:
https://doi.org/10.18413/rrpharmacology.11.809Abstract
Introduction: Despite the high prevalence of depressive disorders, the pathogenesis of depression has not been fully established. Recently, a significant number of works have been published demonstrating mechanisms of development of this pathology that differ from classic monoaminergic theory. In-depth study of such mechanisms can be used as new approaches to the design of more advanced antidepressant drugs.
Materials and Methods: A search of literary sources was conducted in Google Scholar, PubMed and Cochrane databases using keywords in English: “pathogenesis of depression”, “major depressive disorder”, “β-arrestin”, “glutamate depression”, “GABA and depression”, and “neuroinflammation”. The exclusion criteria were sources published earlier than 2015. At the first step, 127 sources were chosen, but after excluding papers containing duplicated or not up-to-date information, only 40 articles were included in the review. The obtained data were briefly summarized in the form of a review article.
Results and Discussion: Translational MRI data indicate that hippocampal volume loss in depression is likely due to a disruption in serotonin-dependent neuroplasticity, which is reversible with SSRI treatment. It is believed that the therapeutic effect of these drugs is not due to a direct effect on symptoms, but rather to a restructuring of neuronal energy metabolism due to increased serotonin levels, which initiates restorative processes in the brain. β-arrestin pathway is alternative to classic in G-proteins, and some data suggest that β-arrestin-2 expression is exactly the key component of fluoxetine’s mechanism of action. Current research reveals structural, functional, and neurochemical abnormalities in GABA- and glutamate-dependent neurons that can lead to impaired signaling in the cerebral cortex and hippocampus. According to current concepts, the molecular mechanisms of these changes are associated with stress-induced excitotoxic effects, occurring against a background of elevated levels of adrenal glucocorticoids and inflammatory cytokines. Current data confirm the key role of neuroinflammation in the development of depression. Animal models of depression consistently show elevated levels of proinflammatory cytokines (IL-1β, IL-6, TNF-α). Multiple psychosocial stressors have been shown to accelerate the development of neuroinflammation, which, in turn, contributes to the progression of major depressive disorder.
Conclusion: Despite a significant amount of research in this area, the role of additional factors in the pathogenesis of depression continues to be actively explored. Established pathogenetic models do not fully explain the disorder’s clinical heterogeneity, and current research focuses on clarifying the contribution of less studied elements, including immune dysfunction, oxidative stress, and the complex interactions between various neurotransmitter systems that go beyond the classical monoamine hypothesis.
Graphical Abstract
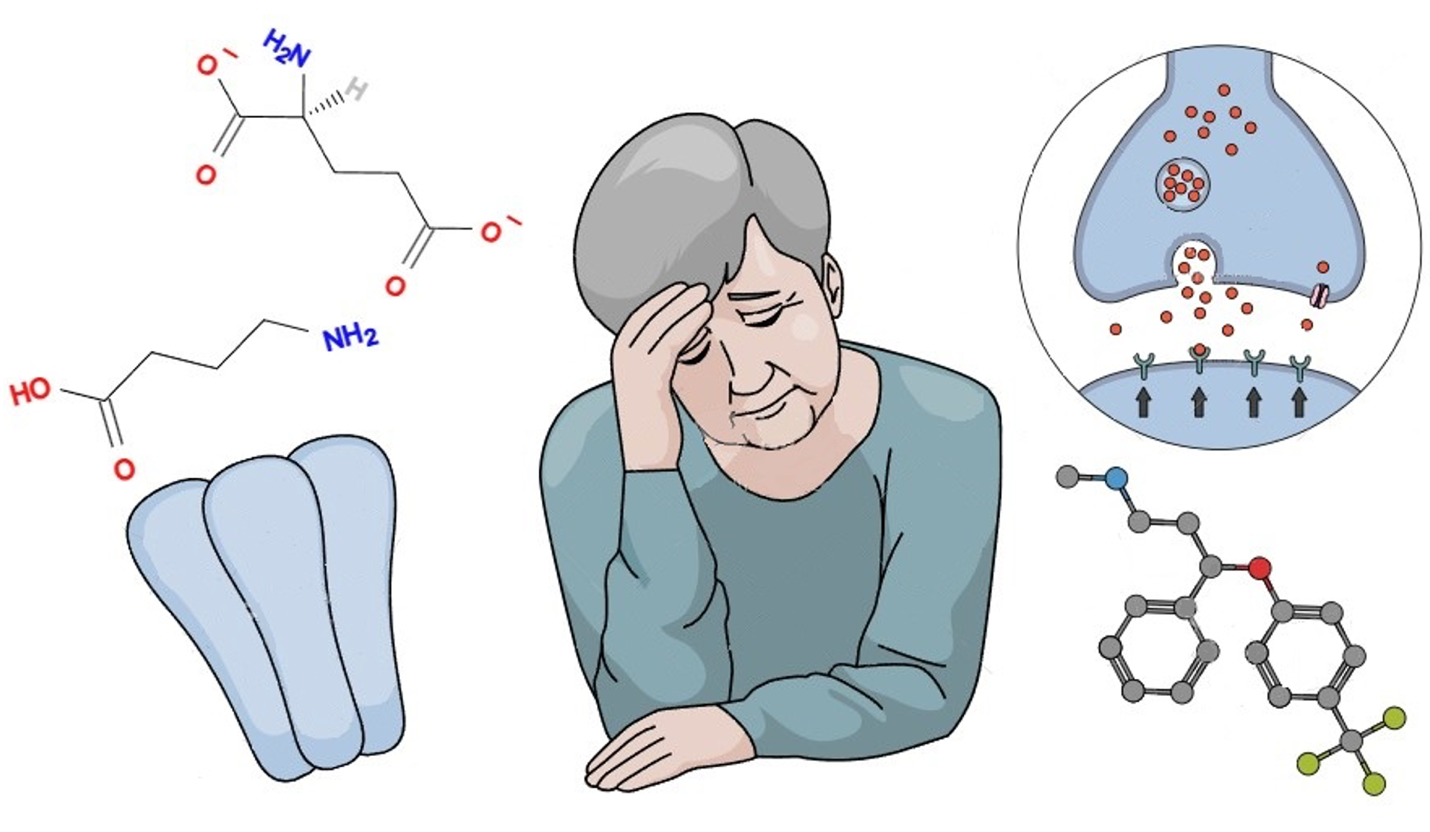
Keywords:
GABA, serotonin, glutamate, β-arrestin, depression, neuroinflammationReferences
Abdallah CG, Jiang L, De Feyter HM, Fasula M, Krystal JH, Rothman DL, Mason GF, Sanacora G (2014) Glutamate metabolism in major depressive disorder. The American Journal of Psychiatry 171(12): 1320–1327. https://doi.org/10.1176/appi.ajp.2014.14010067 [PubMed]
Alexander RC (2017) The potential efficacy of GABAB antagonists in depression. Current Opinion in Pharmacology 35: 101–104. https://doi.org/10.1016/j.coph.2017.07.009 [PubMed]
Andrews PW, Bharwani A, Lee KR, Fox M, Thomson JAJr (2015) Is serotonin an upper or a downer? The evolution of the serotonergic system and its role in depression and the antidepressant response. Neuroscience and Biobehavioral Reviews 51: 164–188. https://doi.org/10.1016/j.neubiorev.2015.01.018 [PubMed]
Belujon P, Grace AA (2017) Dopamine system dysregulation in major depressive disorders. The International Journal of Neuropsychopharmacology 20(12): 1036–1046. https://doi.org/10.1093/ijnp/pyx056 [PubMed]
Borbély É, Simon M, Fuchs E, Wiborg O, Czéh B, Helyes Z (2022) Novel drug developmental strategies for treatment-resistant depression. British Journal of Pharmacology 179(6): 1146–1186. https://doi.org/10.1111/bph.15753 [PubMed]
Cahill TJ, Thomsen AR, Tarrasch JT, Plouffe B, Nguyen AH, Yang F, Huang LY, Kahsai, AW, Bassoni DL, Gavino BJ, Lamerdin JE, Triest S, Shukla AK, Berger B, Little J, Antar A, Blanc A, Qu CX, Chen X, Kawakami K, Lefkowitz RJ (2017) Distinct conformations of GPCR-β-arrestin complexes mediate desensitization, signaling, and endocytosis. Proceedings of the National Academy of Sciences of the United States of America 114(10): 2562–2567. https://doi.org/10.1073/pnas.1701529114 [PubMed]
Cui R (2015) Editorial: A systematic review of depression. Current Neuropharmacology 13(4): 480. https://doi.org/10.2174/1570159x1304150831123535 [PubMed]
de Sousa RT, Loch AA, Carvalho AF, Brunoni AR, Haddad MR, Henter ID, Zarate CA, Machado-Vieira R (2017) Genetic studies on the tripartite glutamate synapse in the pathophysiology and therapeutics of mood disorders. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 42(4): 787–800. https://doi.org/10.1038/npp.2016.149 [PubMed]
Dean RL, Hurducas C, Hawton K, Spyridi S, Cowen PJ, Hollingsworth S, Marquardt T, Barnes A, Smith R, McShane R, Turner EH, Cipriani A (2021) Ketamine and other glutamate receptor modulators for depression in adults with unipolar major depressive disorder. The Cochrane Database of Systematic Reviews 9(9): CD011612. https://doi.org/10.1002/14651858.CD011612.pub3 [PubMed]
Dell’Osso L, Carmassi C, Mucci F, Marazziti D (2016) Depression, serotonin and tryptophan. Current Pharmaceutical Design 22(8): 949–954. https://doi.org/10.2174/1381612822666151214104826 [PubMed]
Duman RS, Sanacora G, Krystal JH (2019) Altered connectivity in depression: GABA and glutamate neurotransmitter deficits and reversal by novel treatments. Neuron 102(1): 75–90. https://doi.org/10.1016/j.neuron.2019.03.013 [PubMed]
Fan J, Guo F, Mo R, Chen LY, Mo JW, Lu CL, Ren J, Zhong QL, Kuang XJ, Wen YL, Gu TT, Liu JM, Li SJ, Fang YY, Zhao C, Gao TM, Cao X (2023) O-GlcNAc transferase in astrocytes modulates depression-related stress susceptibility through glutamatergic synaptic transmission. The Journal of Clinical Investigation 133(7): e160016. https://doi.org/10.1172/JCI160016 [PubMed]
Fee C, Banasr M, Sibille E (2017) Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: Cortical microcircuit and therapeutic perspectives. Biological Psychiatry 82(8): 549–559. https://doi.org/10.1016/j.biopsych.2017.05.024 [PubMed]
Gerhard DM, Wohleb ES, Duman RS (2016) Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discovery Today 21(3): 454–464. https://doi.org/10.1016/j.drudis.2016.01.016 [PubMed]
Grace AA (2016) Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nature Reviews. Neuroscience 17(8): 524–532. https://doi.org/10.1038/nrn.2016.57 [PubMed]
Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M (2015) Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain, Behavior, and Immunity 49: 206–215. https://doi.org/10.1016/j.bbi.2015.06.001 [PubMed]
Harsanyi S, Kupcova I, Danisovic L, Klein M (2022) Selected biomarkers of depression: What are the effects of cytokines and inflammation? International Journal of Molecular Sciences 24(1): 578. https://doi.org/10.3390/ijms24010578[PubMed]
Hassamal S (2023) Chronic stress, neuroinflammation, and depression: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Frontiers in Psychiatry 14: 1130989. https://doi.org/10.3389/fpsyt.2023.1130989[PubMed]
Henter ID, de Sousa RT, Zarate CA (2018) Glutamatergic modulators in depression. Harvard Review of Psychiatry 26(6): 307–319. https://doi.org/10.1097/HRP.0000000000000183 [PubMed]
Hess EM, Riggs LM, Michaelides M, Gould TD (2022) Mechanisms of ketamine and its metabolites as antidepressants. Biochemical Pharmacology 197: 114892. https://doi.org/10.1016/j.bcp.2021.114892 [PubMed]
Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, Rebusi N, Heshmati M, Aleyasin H, Warren BL, Lebonté B, Horn S, Lapidus KA, Stelzhammer V, Wong EH, Bahn S, Krishnan V, Bolaños-Guzman CA, Murrough JW, Merad M, Russo SJ (2014) Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proceedings of the National Academy of Sciences of the United States of America 111(45): 16136–16141. https://doi.org/10.1073/pnas.1415191111 [PubMed]
Hodes GE, Kana V, Menard C, Merad M, Russo SJ (2015) Neuroimmune mechanisms of depression. Nature Neuroscience 18(10): 1386–1393. https://doi.org/10.1038/nn.4113 [PubMed]
Köhler-Forsberg O, N Lydholm C, Hjorthøj C, Nordentoft M, Mors O, Benros ME (2019) Efficacy of anti-inflammatory treatment on major depressive disorder or depressive symptoms: meta-analysis of clinical trials. Acta Psychiatrica Scandinavica 139(5): 404–419. https://doi.org/10.1111/acps.13016 [PubMed]
Kraus C, Castrén E, Kasper S, Lanzenberger R. (2017). Serotonin and neuroplasticity – Links between molecular, functional and structural pathophysiology in depression. Neuroscience and Biobehavioral Reviews 77: 317–326. https://doi.org/10.1016/j.neubiorev.2017.03.007 [PubMed]
Kverno KS, & Mangano E (2021) Treatment-resistant depression: Approaches to treatment. Journal of Psychosocial Nursing and Mental Health Services 59(9): 7–11. https://doi.org/10.3928/02793695-20210816-01 [PubMed]
Kumari P, Srivastava A, Banerjee R, Ghosh E, Gupta P, Ranjan R, Chen X, Gupta B, Gupta C, Jaiman D, Shukla AK (2016) Functional competence of a partially engaged GPCR-β-arrestin complex. Nature Communications 7: 13416. https://doi.org/10.1038/ncomms13416 [PubMed]
Kumari P, Srivastava A, Ghosh E, Ranjan R, Dogra S, Yadav PN, Shukla AK (2017) Core engagement with β-arrestin is dispensable for agonist-induced vasopressin receptor endocytosis and ERK activation. Molecular Biology of the Cell 28(8): 1003–1010. https://doi.org/10.1091/mbc.E16-12-0818 [PubMed]
Li J, Chen L, Li G, Chen X, Hu S, Zheng L, Luria V, Lv J, Sun Y, Xu Y, Yu Y (2020) Sub-acute treatment of curcumin derivative J147 ameliorates depression-like behavior through 5-HT1A-mediated cAMP signaling. Frontiers in neuroscience 14: 701. https://doi.org/10.3389/fnins.2020.00701 [PubMed]
Li H, Cui L, Li J, Liu Y, Chen Y (2021) Comparative efficacy and acceptability of neuromodulation procedures in the treatment of treatment-resistant depression: a network meta-analysis of randomized controlled trials. Journal of Affective Disorders 287: 115–124. https://doi.org/10.1016/j.jad.2021.03.019 [PubMed]
Li Z, Ruan M, Chen J, Fang Y (2021) Major depressive disorder: Advances in neuroscience research and translational applications. Neuroscience Bulletin 37(6): 863–880. https://doi.org/10.1007/s12264-021-00638-3 [PubMed]
Lener MS, Niciu MJ, Ballard ED, Park M, Park LT, Nugent AC, Zarate CA (2017). Glutamate and gamma-aminobutyric acid systems in the pathophysiology of major depression and antidepressant response to ketamine. Biological Psychiatry 81(10): 886–897. https://doi.org/10.1016/j.biopsych.2016.05.005 [PubMed]
Ménard C, Hodes GE, Russo SJ (2016) Pathogenesis of depression: Insights from human and rodent studies. Neuroscience 321: 138–162. https://doi.org/10.1016/j.neuroscience.2015.05.053 [PubMed]
Mineur YS, Cahuzac EL, Mose TN, Bentham MP, Plantenga ME, Thompson DC, Picciotto MR (2018) Interaction between noradrenergic and cholinergic signaling in amygdala regulates anxiety- and depression-related behaviors in mice. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology 43(10): 2118–2125. https://doi.org/10.1038/s41386-018-0024-x [PubMed]
Smith JS, Lefkowitz RJ, Rajagopal S (2018) Biased signalling: From simple switches to allosteric microprocessors. Nature Reviews. Drug Discovery 17(4): 243–260. https://doi.org/10.1038/nrd.2017.229 [PubMed]
Tejeda-Martínez AR, Ramos-Molina AR, Brand-Rubalcava PA, Flores-Soto ME (2024) Involvement of serotonergic receptors in depressive processes and their modulation by β-arrestins: A review. Medicine 103(28): e38943. https://doi.org/10.1097/MD.0000000000038943 [PubMed]
Troubat R, Barone P, Leman S, Desmidt T, Cressant A, Atanasova B, Brizard B, El Hage W, Surget A, Belzung C, Camus V (2021) Neuroinflammation and depression: A review. The European Journal of Neuroscience 53(1): 151–171. https://doi.org/10.1111/ejn.14720 [PubMed]
Wang H, He Y, Sun Z, Ren S, Liu M, Wang G, Yang J (2022) Microglia in depression: an overview of microglia in the pathogenesis and treatment of depression. Journal of Neuroinflammation 19(1): 132. https://doi.org/10.1186/s12974-022-02492-0 [PubMed]
Won E, Na KS, Kim YK (2021) Associations between melatonin, neuroinflammation, and brain alterations in depression. International Journal of Molecular Sciences 23(1): 305. https://doi.org/10.3390/ijms23010305 [PubMed]
Wu A, Zhang J (2023) Neuroinflammation, memory, and depression: new approaches to hippocampal neurogenesis. Journal of Neuroinflammation 20(1): 283. https://doi.org/10.1186/s12974-023-02964-x [PubMed]
Zorumski CF, Paul SM, Covey DF, Mennerick S (2019) Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiology of Stress 11: 100196. https://doi.org/10.1016/j.ynstr.2019.100196 [PubMed]
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Spasov AA, Maltsev DV, Maltseva MO

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

