The therapeutic potential of mesenchymal stem cells in COVID-19: Present and future
DOI:
https://doi.org/10.18413/rrpharmacology.9.10031Abstract
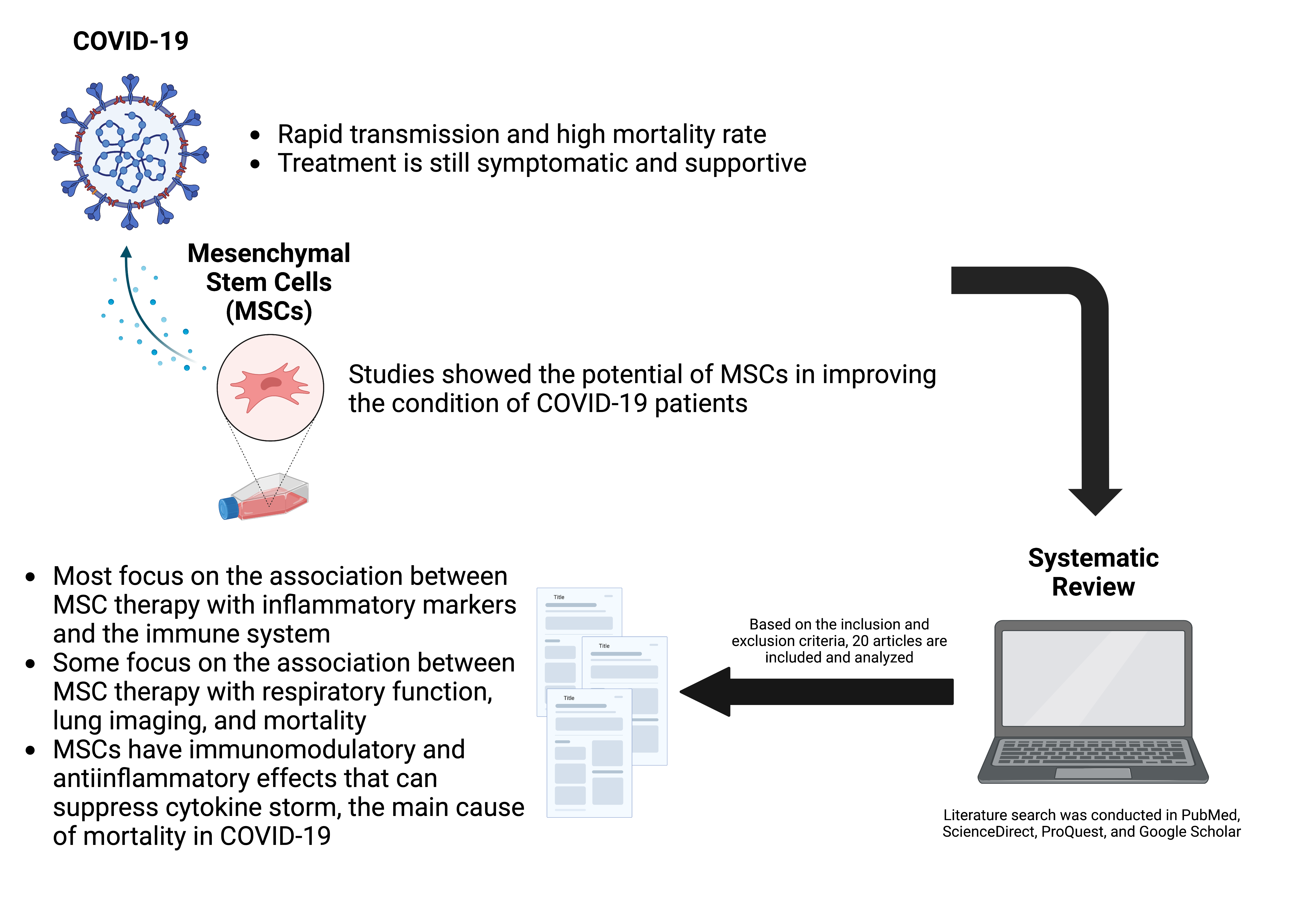
Introduction: Coronavirus disease 2019 (COVID-19) was first reported in 2019 and has since become a health concern due to its rapid spread and high mortality rate. With the discovery of vaccines, there has been a reduction in disease occurrence, transmission, mortality, and morbidity in a population. However, with the emergence of newvariants, the available vaccines show varying efficiencies depending on the population and variants, while the present drugs may lose their effectiveness, hence the urgent need to explore effective therapies. Mesenchymal stemcells (MSCs) have been widely studied for their anti-inflammatory and immunomodulatory effects as COVID-19treatment and shown their potential to improve the condition of COVID-19 patients. This systematic review aims to assess the therapeutic potential of MSCs as anti-inflammatory and immunomodulatory agent in COVID-19.
Materials and Methods: A literature search is performed on PubMed, ScienceDirect, ProQuest, and Google Scholar and potentially relevant studies to review, based on the inclusion and exclusion criteria we have determined.We identified 14,090 publications from our search and excluded duplicates as well as irrelevant studies from title,abstract, and full-text screening. Data extraction and analysis were then performed in the 20 eligible studies.
Results and Discussion: Results show that MSCs improve immune system dysregulation throughimmunomodulatory and anti-inflammatory effects, through reducing blood C-reactive protein (CRP) and IL-6 levels.
Conclusion: We conclude that MSC is one of the promising treatments in COVID-19 regardless of variants.
Graphical Abstract
Keywords:
anti-inflammatory, cell therapy, immunomodulatory, inflammation, virusReferences
Adas G, Cukurova Z, Yasar KK, Yilmaz R, Isiksacan N, Kasapoglu P, Yesilbag Z, Koyuncu ID, Karaoz E (2021) The systematic effect of mesenchymal stem cell therapy in critical covid-19 patients: A prospective double controlled trial. Cell Transplantation 30: 1–14. https://doi.org/10.1177/09636897211024942 [PubMed] [PMC]
Alencar CH, Cavalcanti LPG, Almeida MM, Barbosa PPL, Cavalcante KKS, Melo DN, de Brito Alves BCF, Heukelbach J (2021) High effectiveness of SARS-CoV-2 vaccines in reducing COVID-19-related deaths in over 75-year-olds, ceará state, Brazil. Tropical Medicine and Infectious Disease 6(3): 129. https://doi.org/10.3390/tropicalmed6030129 [PubMed] [PMC]
Alatyyat SM, Alasmari HM, Aleid OA, Abdel-maksoud MS, Elsherbiny N (2020) Umbilical cord stem cells: Background, processing and applications. Tissue and Cell 65: 101351. https://doi.org/10.1016/j.tice.2020.101351[PubMed]
Atluri S, Manchikanti L, Hirsch JA (2020) Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically Ill COVID-19 patients: The Case for compassionate use. Pain Physician 23(2): E71–E83. [PubMed]
Bacca E, Digaetano M, Meschiari M, Franceschini E, Menozzi M, Cuomo G, Mussini C (2021) Immunomodulation for the management of severe SARS-CoV2 infections. State of the art and review of the literature. Biochemical and Biophysical Research Communications 538: 151–155. https://doi.org/10.1016/j.bbrc.2020.11.084 [PubMed] [PMC]
Bassetti M, Vena A, Giacobbe DR (2020) The novel Chinese coronavirus (2019-nCoV) infections: Challenges for fighting the storm. European Journal of Clinical Investigation 50(3): e13209. https://doi.org/10.1111/eci.13209 [PubMed] [PMC]
Becerra J, Duran I (2021) Inflammation, a common mechanism in frailty and COVID-19, and stem cells as a therapeutic approach. Stem Cells Translational Medicine 10(11): 1482–1490. https://doi.org/10.1002/sctm.21-0074 [PubMed] [PMC]
Beeraka NM, Sadhu SP, Madhunapantula SV, Rao Pragada R, Svistunov AA, Nikolenko VN, Mikhaleva LM, Aliev G (2020) Strategies for targeting SARS CoV-2: Small molecule inhibitors-the current status. Frontiers in Immunology 11: 552925. https://doi.org/10.3389/fimmu.2020.552925 [PubMed] [PMC]
Chouw A, Milanda T, Sartika CR, Kirana MN, Halim D, Faried A (2022) Potency of mesenchymal stem cell and its secretome in treating COVID-19. Regenerative Engineering and Translational Medicine 8(1): 43–54. https://doi.org/10.1007/s40883-021-00202-5 [PubMed] [PMC]
COVID-ICU Group (2021) Clinical characteristics and day‑90 outcomes of 4244 critically ill adults with COVID‑19: A prospective cohort study. Intensive Care Medicine 47(1): 60–73. https://doi.org/10.1007/s00134-020-06294-x [PubMed] [PMC]
Danchuk S, Ylostalo JH, Hossain F, Sorge R, Ramsey A, Bonvillain RW, Lasky JA, Bunnell BA, Welsh DA, Prockop DJ, Sullivan DE (2011) Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-α-induced protein 6. Stem Cell Research & Therapy 2(3): 27. https://doi.org/10.1186/scrt68 [PubMed] [PMC]
Daneshmandi L, Shah S, Jafari T, Bhattacharjee M, Momah D, Saveh-Shemshaki N, Lo KW, Laurencin CT (2020) Emergence of the stem cell secretome in regenerative engineering. Trends in Biotechnology 38(12): 1373–1384. https://doi.org/10.1016/j.tibtech.2020.04.013 [PubMed] [PMC]
de Witte SFH, Luk F, Sierra Parraga JM, Gargesha M, Merino A, Korevaar SS, Shankar AS, O'Flynn L, Elliman SJ, Roy D, Betjes MGH, Newsome PN, Baan CC, Hoogduijn MJ (2018) Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cells 36(4): 602–615. https://doi.org/10.1002/stem.2779 [PubMed]
Dilogo IH, Aditianingsih D, Sugiarto A, Burhan E, Damayanti T, Sitompul PA, Mariana N, Antarianto RD, Liem IK, Kispa T, Mujadid F, Novialdi N, Luviah E, Kurniawati T, Lubis AMT, Rahmatika D (2021) Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: A randomized controlled trial. Stem Cells Translational Medicine 10(9): 1279–1287. https://doi.org/10.1002/sctm.21-0046 [PubMed] [PMC]
Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ (2012) Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Frontiers in Immunology 3: 297. https://doi.org/10.3389/fimmu.2012.00297 [PubMed] [PMC]
Fathi-Kazerooni M, Fattah-Ghazi S, Darzi M, Makarem J, Nasiri R, Salahshour F, Dehghan-Manshadi SA, Kazemnejad S (2022) Safety and efficacy study of allogeneic human menstrual blood stromal cells secretome to treat severe COVID‑19 patients: clinical trial phase I & II. Stem Cell Research & Therapy 13(1): 96. https://doi.org/10.1186/s13287-022-02771-w[PubMed] [PMC]
Feng G, Shi L, Huang T, Ji N, Zheng Y, Lin H, Niu C, Wang Y, Li R, Huang M, Chen X, Shu L, Wu M, Deng K, Wei J, Wang X, Cao Y, Yan J (2021) Human umbilical cord mesenchymal stromal cell treatment of severe COVID-19 patients: a 3-month follow-up study following hospital discharge. Stem Cells and Development 30(15): 773–781. https://doi.org/10.1089/scd.2021.0015 [PubMed]
Feng Y, Huang J, Wu J, Xu Y, Chen B, Jiang L, Xiang H, Peng Z, Wang X (2020) Safety and feasibility of umbilical cord mesenchymal stem cells in patients with COVID-19 pneumonia: A pilot study. Cell Proliferation 53(12): e12947. https://doi.org/10.1111/cpr.12947 [PubMed] [PMC]
Galipeau J, Sensébé L (2018) Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell 22(6): 824–833. https://doi.org/10.1016/j.stem.2018.05.004 [PubMed] [PMC]
Garcia LF (2020) Immune response, inflammation, and the clinical spectrum of COVID-19. Frontiers in Immunology 11: 1441. https://doi.org/10.3389/fimmu.2020.01441 [PubMed] [PMC]
Gwam C, Mohammed N, Ma X (2021) Stem cell secretome, regeneration, and clinical translation: a narrative review. Annals of Translational Medicine 9(1): 70. https://doi.org/10.21037/atm-20-5030 [PubMed] [PMC]
Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y (2020) The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Military Medical Research 7(1): 11. https://doi.org/10.1186/s40779-020-00240-0 [PubMed] [PMC]
Häberle H, Magunia H, Lang P, Gloeckner H, Körner A, Koeppen M, Backchoul T, Malek N, Handgretinger R, Rosenberger P, Mirakaj V (2021) Mesenchymal stem cell therapy for severe COVID-19 ARDS. Journal of Intensive Care Medicine 36(6): 681–688. https://doi.org/10.1177/0885066621997365 [PubMed] [PMC]
Halim C, Mirza AF, Sari MI (2022) The association between TNF-α, IL-6, and vitamin D levels and COVID-19 severity and mortality: A Systematic review and meta-analysis. Pathogens 11(2): 195. https://doi.org/10.3390/pathogens11020195[PubMed] [PMC]
Han Y, Yang J, Fang J, Zhou Y, Candi E, Wang J, Hua D, Shao C, Shi Y (2022) The secretion profile of mesenchymal stem cells and potential applications in treating human diseases. Signal Transduction and Targeted Therapy 7(1): 92. https://doi.org/10.1038/s41392-022-00932-0 [PubMed] [PMC]
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223): 497–506. https://doi.org/10.1016/S0140-6736(20)30183-5 [PubMed] [PMC]
Huang I, Pranata R (2020) Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. Journal of Intensive Care 8: 36. https://doi.org/10.1186/s40560-020-00453-4 [PubMed] [PMC]
Huang YZ, Kuan CC (2022) Vaccination to reduce severe COVID-19 and mortality in COVID-19 patients: a systematic review and meta-analysis. European Review for Medical and Pharmacological Sciences 26(5): 1770–1776. https://doi.org/10.26355/eurrev_202203_28248 [PubMed]
Irmak DK, Karaoz E (2021) Cellular therapy as promising choice of treatment for COVID-19, and stem cells as a therapeutic approach. IntechOpen. https://doi.org/10.5772/intechopen.96900
Karakaş N, Üçüncüoğlu S, Uludağ D, Karaoğlan BS, Shah K, Öztürk G (2022) Mesenchymal stem cell-based COVID-19 therapy: Bioengineering perspectives. Cells 11(3): 465. https://doi.org/10.3390/cells11030465 [PubMed] [PMC]
Karyana M, Djaharuddin I, Rif'ati L, Arif M, Choi MK, Angginy N, Yoon A, Han J, Josh F, Arlinda D, Narulita A, Muchtar F, Bakri RA, Irmansyah S (2022) Safety of DW-MSC infusion in patients with low clinical risk COVID-19 infection: a randomized, double-blind, placebo-controlled trial. Stem Cell Research & Therapy 13(1): 134. https://doi.org/10.1186/s13287-022-02812-4 [PubMed] [PMC]
Kaushal K, Kaur H, Sarma P, Bhattacharyya A, Sharma DJ, Prajapat M, Pathak M, Kothari A, Kumar S, Rana S, Kaur M, Prakash A, Mirza AA, Panda PK, Vivekanandan S, Omar BJ, Medhi B, Naithani M (2022) Serum ferritin as a predictive biomarker in COVID-19. A systematic review, meta-analysis and meta-regression analysis. Journal of Critical Care 67: 172–181. https://doi.org/10.1016/j.jcrc.2021.09.023 [PubMed] [PMC]
Kavianpour M, Saleh M, Verdi J (2020) The role of mesenchymal stromal cells in immune modulation of COVID-19: focus on cytokine storm. Stem Cell Research & Therapy 11(1): 404. https://doi.org/10.1186/s13287-020-01849-7[PubMed] [PMC]
Kean TJ, Lin P, Caplan AI, Dennis JE (2013) MSCs: Delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells International 2013: 732742. https://doi.org/10.1155/2013/732742 [PubMed] [PMC]
Khoury M, Alcayaga-Miranda F, Illanes SE, Figueroa FE (2014) The promising potential of menstrual stem cells for antenatal diagnosis and cell therapy. Frontiers in Immunology 5: 205. https://doi.org/10.3389/fimmu.2014.00205[PubMed] [PMC]
Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV (2013) Wharton’s jelly-derived mesenchymal stem cells: Phenotypic characterization and optimizing their therapeutic potential for clinical applications. International Journal of Molecular Sciences 14(6): 11692–11712. https://doi.org/10.3390/ijms140611692 [PubMed] [PMC]
Lanzoni G, Linetsky E, Correa D, Cayetano SM, Alvarez RA, Kouroupis D, Alvarez Gil A, Poggioli R, Ruiz P, Marttos AC, Hirani K, Bell CA, Kusack H, Rafkin L, Baidal D, Pastewski A, Gawri K, Leñero C, Mantero AMA, Metalonis SW, Wang X, Roque L, Masters B, Kenyon NS, Ginzburg E, Xu X, Tan J, Caplan AI, Glassberg MK, Alejandro R, Ricordi C (2021) Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. STEM CELLS Translational Medicine 10(5): 660–673. https://doi.org/10.1002/sctm.20-0472 [PubMed] [PMC]
Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC (2020) Transplantation of ACE2-mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging and Disease 11(2): 216–228. https://doi.org/10.14336/AD.2020.0228 [PubMed] [PMC]
Levi M, van der Poll T (2017) Coagulation and sepsis. Thrombosis Research 149: 38–44. https://doi.org/10.1016/j.thromres.2016.11.007
Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, Zheng QF, Zhao GB, Ma ZJ (2015) Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Research & Therapy 6(1): 55. https://doi.org/10.1186/s13287-015-0066-5 [PubMed] [PMC]
Li W, Ren G, Huang Y, Su J, Han Y, Li J, Chen X, Cao K, Chen Q, Shou P, Zhang L, Yuan ZR, Roberts AI, Shi S, Le AD, Shi Y (2012) Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death & Differentiation 19(9): 1505–1513. https://doi.org/10.1038/cdd.2012.26 [PubMed] [PMC]
Li Y, Deng Y, Ye L, Sun H, Du S, Huang H, Zeng F, Chen X, Deng G (2021) Clinical significance of plasma D-dimer in COVID-19 mortality. Frontiers in Medicine (Lausanne) 8: 638097. https://doi.org/10.3389/fmed.2021.638097 [PubMed] [PMC]
Liang B, Chen J, Li T, Wu H, Yang W, Li Y, Li J, Yu C, Nie F, Ma Z, Yang M, Xiao M, Nie P, Gao Y, Qian C, Hu M (2020) Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells. Medicine 99(31): e21429. https://doi.org/10.1097/MD.0000000000021429 [PubMed] [PMC]
Liang LL, Kuo HS, Ho HJ, Wu CY (2021) COVID-19 vaccinations are associated with reduced fatality rates: Evidence from cross-county quasi-experiments. Journal of Global Health 11: 05019. https://doi.org/10.7189/jogh.11.05019[PubMed] [PMC]
Liu Y, Yu Y, Zhao Y, He D (2022) Reduction in the infection fatality rate of Omicron variant compared with previous variants in South Africa. International Journal of Infectious Diseases 120: 146–149. https://doi.org/10.1016/j.ijid.2022.04.029 [PubMed] [PMC]
Luan YY, Yin CH, Yao YM (2021) Update advances on C-Reactive protein in COVID-19 and other viral infections. Frontiers in Immunology 12: 720363. https://doi.org/10.3389/fimmu.2021.720363 [PubMed] [PMC]
Melo AKG, Milby KM, Caparroz ALMA, Pinto ACPN, Santos RRP, Rocha AP, Ferreira GA, Souza VA, Valadares LDA, Vieira RMRA, Pileggi GS, Trevisani VFM (2021) Biomarkers of cytokine storm as red flags for severe and fatal COVID-19 cases: A living systematic review and meta-analysis. PLoS One 16(6): e0253894. https://doi.org/10.1371/journal.pone.0253894 [PubMed] [PMC]
Meng F, Xu R, Wang S, Xu Z, Zhang C, Li Y, Yang T, Shi L, Fu J, Jiang T, Huang L, Zhao P, Yuan X, Fan X, Zhang JY, Song J, Zhang D, Jiao Y, Liu L, Zhou C, Maeurer M, Zumla A, Shi M, Wang FS (2020) Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduction and Targeted Therapy 5(1): 172. https://doi.org/10.1038/s41392-020-00286-5 [PubMed] [PMC]
Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, Wang H, Ge W, Bogin V, Chan KW, Thébaud B, Riordan NH (2007) Endometrial regenerative cells: A novel stem cell population. Journal of Translational Medicine 5: 57. https://doi.org/10.1186/1479-5876-5-57 [PubMed] [PMC]
Monsel A, Hauw-Berlemont C, Mebarki M, Heming N, Mayaux J, Tchoumba ON, Diehl JL, Demoule A, Annane D, Marois C, Demeret S, Weiss E, Voiriot G, Fartoukh M, Constantin JM, Mégarbane B, Plantefève G, Malard-Castagnet S, Burrel S, Rosenzwajg M, Tchitchek N, Boucher-Pillet H, Churlaud G, Cras A, Maheux C, Pezzana C, Diallo MH, Ropers J, Menasché P, Larghero J; APHP STROMA–CoV-2 Collaborative Research Group (2022) Treatment of COVID‑19‑associated ARDS with mesenchymal stromal cells: a multicenter randomized double‑blind trial. Critical Care 26(1): 48. https://doi.org/10.1186/s13054-022-03930-4 [PubMed] [PMC]
Montanucci P, Pescara T, Greco A, Francisci D, Basta G, Calafiore R (2021) Microencapsulated Wharton Jelly-derived adult mesenchymal stem cells as a potential new therapeutic tool for patients with COVID-19 disease: an in vitro study. American Journal of Stem Cells 10(3): 36–52. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8449139/ [PubMed] [PMC]
Mounayar M, Kefaloyianni E, Smith B, Solhjou Z, Maarouf OH, Azzi J, Chabtini L, Fiorina P, Kraus M, Briddell R, Fodor W, Herrlich A, Abdi R (2015) PI3kα and STAT1 interplay regulates human mesenchymal stem cell immune polarization. Stem Cells 33(6): 1892–1901. https://doi.org/10.1002/stem [PubMed] [PMC]
Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E (2020) A novel coronavirus emerging in China — key questions for impact assessment. The New England Journal of Medicine 382(8): 692–694. https://doi.org/10.1056/NEJMp2000929 [PubMed]
Nasef A, Chapel A, Mazurier C, Bouchet S, Lopez M, Mathieu N, Sensebé L, Zhang Y, Gorin NC, Thierry D, Fouillard L (2007) Identification of IL-10 and TGF-beta transcripts involved in the inhibition of T-lymphocyte proliferation during cell contact with human mesenchymal stem cells. Gene Expression 13(4-5): 217–226.https://doi.org/10.3727/000000006780666957 [PubMed] [PMC]
Noris M, Benigni A, Remuzzi G (2020) The case of complement activation in COVID-19 multiorgan impact. Kidney International 98(2): 314–322. https://doi.org/10.1016/j.kint.2020.05.013 [PubMed] [PMC]
Ostrand-Rosenberg S, Horn LA, Haile ST (2014) The programmed death-1 immune suppressive pathway: Barrier to anti-tumor immunity. The Journal of Immunology 193(8): 3835–3841. https://doi.org/10.4049/jimmunol.1401572 [PubMed] [PMC]
Que Y, Hu C, Wan K, Hu P, Wang R, Luo J, Li T, Ping R, Hu Q, Sun Y, Wu X, Tu L, Du Y, Chang C, Xu G (2022) Cytokine release syndrome in COVID-19: a major mechanism of morbidity and mortality. International Reviews of Immunology 41(2): 217–230. https://doi.org/10.1080/08830185.2021.1884248 [PubMed] [PMC]
Rabaan AA, Mutair AA, Hajissa K, Alfaraj AH, Al-Jishi JM, Alhajri M, Alwarthan S, Alsuliman SA, Al-Najjar AH, Al Zaydani IA, Al-Absi GH, Alshaikh SA, Alkathlan MS, Almuthree SA, Alawfi A, Alshengeti A, Almubarak FZ, Qashgari MS, Abdalla ANK, Alhumaid S (2022) A comprehensive review on the current vaccines and their efficacies to combat SARS-CoV-2 variants. Vaccines (Basel) 10(10): 1655. https://doi.org/10.3390/vaccines10101655 [PubMed] [PMC]
Rajarshi K, Chatterjee A, Ray S (2020) Combating COVID-19 with mesenchymal stem cell therapy. Biotechnology Reports 26: e00467. https://doi.org/10.1016/j.btre.2020.e00467 [PubMed] [PMC]
Rebelatto CLK, Senegaglia AC, Franck CL, Daga DR, Shigunov P, Stimamiglio MA, Marsaro DB, Schaidt B, Micosky A, de Azambuja AP, Leitão CA, Petterle RR, Jamur VR, Vaz IM, Mallmann AP, Carraro Junior H, Ditzel E, Brofman PRS, Correa A (2022) Safety and long-term improvement of mesenchymal stromal cell infusion in critically COVID-19 patients: a randomized clinical trial. Stem Cell Research & Therapy 13(1): 122. https://doi.org/10.1186/s13287-022-02796-1 [PubMed] [PMC]
Rocha JLM, de Oliveira WCF, Noronha NC, Dos Santos NCD, Covas DT, Picanço-Castro V, Swiech K, Malmegrim KCR (2021) Mesenchymal stromal cells in viral infections: Implications for COVID-19. Stem Cell Reviews and Reports 17(1): 71–93. https://doi.org/10.1007/s12015-020-10032-7 [PubMed] [PMC]
Ruan Q, Yang K, Wang W, Jiang L, Song J (2020) Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Medicine 46(5): 846–848. https://doi.org/10.1007/s00134-020-05991-x [PubMed] [PMC]
Rubin R (2021) COVID-19 vaccines vs variants-determining how much immunity is enough. Journal of the American Medical Association 325(13): 1241–1243. https://doi.org/10.1001/jama.2021.3370 [PubMed]
Saleh M, Vaezi AA, Aliannejad R, Sohrabpour AA, Kiaei SZF, Shadnoush M, Siavashi V, Aghaghazvini L, Khoundabi B, Abdoli S, Chahardouli B, Seyhoun I, Alijani N, Verdi J (2021) Cell therapy in patients with COVID-19 using Wharton’s jelly mesenchymal stem cells: a phase 1 clinical trial. Stem Cell Research & Therapy 12(1): 410. https://doi.org/10.1186/s13287-021-02483-7 [PubMed] [PMC]
Sari DK, Amelia R, Dharmajaya R, Sari LM, Fitri NK (2021) Positive correlation between general public knowledge and attitudes regarding COVID‑19 outbreak 1 month after first cases reported in Indonesia. Journal of Community Health 46(1): 182–189. https://doi.org/10.1007/s10900-020-00866-0 [PubMed] [PMC]
Sengupta V, Sengupta S, Lazo A, Jr., Woods P, Nolan A, Bremer N (2020) Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells and Development 29(12): 747–754. https://doi.org/10.1089/scd.2020.0080 [PubMed] [PMC]
Shi L, Huang H, Lu X, Yan X, Jiang X, Xu R, Wang S, Zhang C, Yuan X, Xu Z, Huang L, Fu JL, Li Y, Zhang Y, Yao WQ, Liu T, Song J, Sun L, Yang F, Zhang X, Zhang B, Shi M, Meng F, Song Y, Yu Y, Wen J, Li Q, Mao Q, Maeurer M, Zumla A, Yao C, Xie WF, Wang FS (2021) Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduction and Targeted Therapy 6(1): 58. https://doi.org/10.1038/s41392-021-00488-5 [PubMed] [PMC]
Shi L, Yuan X, Yao W, Wang S, Zhang C, Zhang B, Song J, Huang L, Xu Z, Fu JL, Li Y, Xu R, Li TT, Dong J, Cai J, Li G, Xie Y, Shi M, Li Y, Zhang Y, Xie WF, Wang FS (2022) Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine 75: 103789. https://doi.org/10.1016/j.ebiom.2021.103789 [PubMed] [PMC]
Shu L, Niu C, Li R, Huang T, Wang Y, Huang M, Ji N, Zheng Y, Chen X, Shi L, Wu M, Deng K, Wei J, Wang X, Cao Y, Yan J, Feng G (2020) Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Research & Therapy 11(1): 361. https://doi.org/10.1186/s13287-020-01875-5 [PubMed] [PMC]
Sinclair K, Yerkovich ST, Chambers DC (2013) Mesenchymal stem cells and the lung. Respirology 18(3): 397–411. https://doi.org/10.1111/resp.12050 [PubMed]
Strasser A, Jost PJ, Nagata S (2009) The many roles of FAS receptor signaling in the immune system. Immunity 30(2): 180–192. https://doi.org/10.1016/j.immuni.2009.01.001 [PubMed] [PMC]
Stringer D, Braude P, Myint PK, Evans L, Collins JT, Verduri A, Quinn TJ, Vilches-Moraga A, Stechman MJ, Pearce L, Moug S, McCarthy K, Hewitt J, Carter B (2021) COPE Study collaborators. The role of C-reactive protein as a prognostic marker in COVID-19. International Journal of Epidemiology 50(2): 420–429. https://doi.org/10.1093/ije/dyab012[PubMed] [PMC]
Tang L, Jiang Y, Zhu M, Chen L, Zhou X, Zhou C, Ye P, Chen X, Wang B, Xu Z, Zhang Q, Xu X, Gao H, Wu X, Li D, Jiang W, Qu J, Xiang C, Li L (2020) Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Frontiers in Medicine 14(5): 664–673. https://doi.org/10.1007/s11684-020-0810-9 [PubMed] [PMC]
Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R (2017) Mesenchymal stem cell secretome: Toward cell-free therapeutic strategies in regenerative medicine. International Journal of Molecular Sciences 18(9): 1852. https://doi.org/10.3390/ijms18091852 [PubMed] [PMC]
Weiss ARR, Lee O, Eggenhofer E, Geissler E, Korevaar SS, Soeder Y, Schlitt HJ, Geissler EK, Hoogduijn MJ, Dahlke MH (2020) Differential effects of heat-inactivated, secretome-deficient MSC and metabolically active MSC in sepsis and allogenic heart transplantation. Stem Cells (Dayton, Ohio) 38(6): 797–807. https://doi.org/10.1002/stem.3165 [PubMed]
Xu R, Feng Z, Wang FS (2022) Mesenchymal stem cell treatment for COVID-19. EBioMedicine. 77: 103920. https://doi.org/10.1016/j.ebiom.2022 [PubMed] [PMC]
Xu X, Jiang W, Chen L, Xu Z, Zhang Q, Zhu M, Ye P, Li H, Yu L, Zhou X, Zhou C, Chen X, Zheng X, Xu K, Cai H, Zheng S, Jiang W, Wu X, Li D, Chen L, Luo Q, Wang Y, Qu J, Li Y, Zheng W, Jiang Y, Tang L, Xiang C, Li L (2021) Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: An exploratory clinical trial. Clinical and Translational Medicine 11(2): e297. https://doi.org/10.1002/ctm2.297 [PubMed] [PMC]
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. The Lancet Respiratory Medicine 8(5): 475–481. https://doi.org/10.1016/S2213-2600(20)30079-5 [PubMed] [PMC]
Yin J, Li C, Ye C, Ruan Z, Liang Y, Li Y, Wu J, Luo Z (2022) Advances in the development of therapeutic strategies against COVID-19 and perspectives in the drug design for emerging SARS-CoV-2 variants. Computational and Structural Biotechnology Journal 20: 824–837. https://doi.org/10.1016/j.csbj.2022.01.026 [PubMed] [PMC]
Zhang C, Wu Z, Li JW, Zhao H, Wang GQ (2020) Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. International Journal of Antimicrobial Agents 55(5): 105954. https://doi.org/10.1016/j.ijantimicag.2020.105954 [PubMed] [PMC]
Zhang X, Wu S, Wu B, Yang Q, Chen A, Li Y, Zhang Y, Pan T, Zhang H, He X (2021) SARS-CoV-2 Omicron strain exhibits potent capabilities for immune evasion and viral entrance. Signal Transduction and Targeted Therapy 6(1): 430. https://doi.org/10.1038/s41392-021-00852-5 [PubMed] [PMC]
Zhou YW, Xie Y, Tang LS, Pu D, Zhu YJ, Liu JY, Ma XL (2021) Therapeutic targets and interventional strategies in COVID-19: mechanisms and clinical studies. Signal Transduction and Targeted Therapy 6(1): 317. https://doi.org/10.1038/s41392-021-00733-x [PubMed] [PMC]
Zhu R, Yan T, Feng Y, Liu Y, Cao H, Peng G, Yang Y, Xu Z, Liu J, Hou W, et al (2021) Mesenchymal stem cell treatment improves outcome of COVID-19 patients via multiple immunomodulatory mechanisms. Cell Research 31(12): 1244–1262. https://doi.org/10.1038/s41422-021-00573-y [PubMed] [PMC]
Zhu YG, Shi MM, Monsel M, Dai C, Dong XC, Shen H, Li SK, Chang J, Xu CL, Li P, Wang J, Shen M-P, Ren CJ, Chen DC, Qu JM (2022) Nebulized exosomes derived from allogenic adipose tissue mesenchymal stromal cells in patients with severe COVID-19: A pilot study. Stem Cell Research & Therapy 13(1): 220. https://doi.org/10.1186/s13287-022-02900-5[PubMed] [PMC]
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Research Results in Pharmacology

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

