Эффективность тетрапептида HAEE: инновационный подход к лечению болезни Альцгеймера в эксперименте
DOI:
https://doi.org/10.18413/rrpharmacology.10.548Аннотация
Аннотация: Болезнь Альцгеймера (БА) представляет собой наиболее распространённую нейродегенеративную патологию, характеризующуюся прогрессирующим снижением когнитивных функций. По данным «Международной организации по борьбе с болезнью Альцгеймера», в 2009 году в мире было зарегистрировано 36 миллионов случаев БА, и прогнозируется, что к 2030 году их число увеличится до 66 миллионов, а к 2050 году — до 115 миллионов.
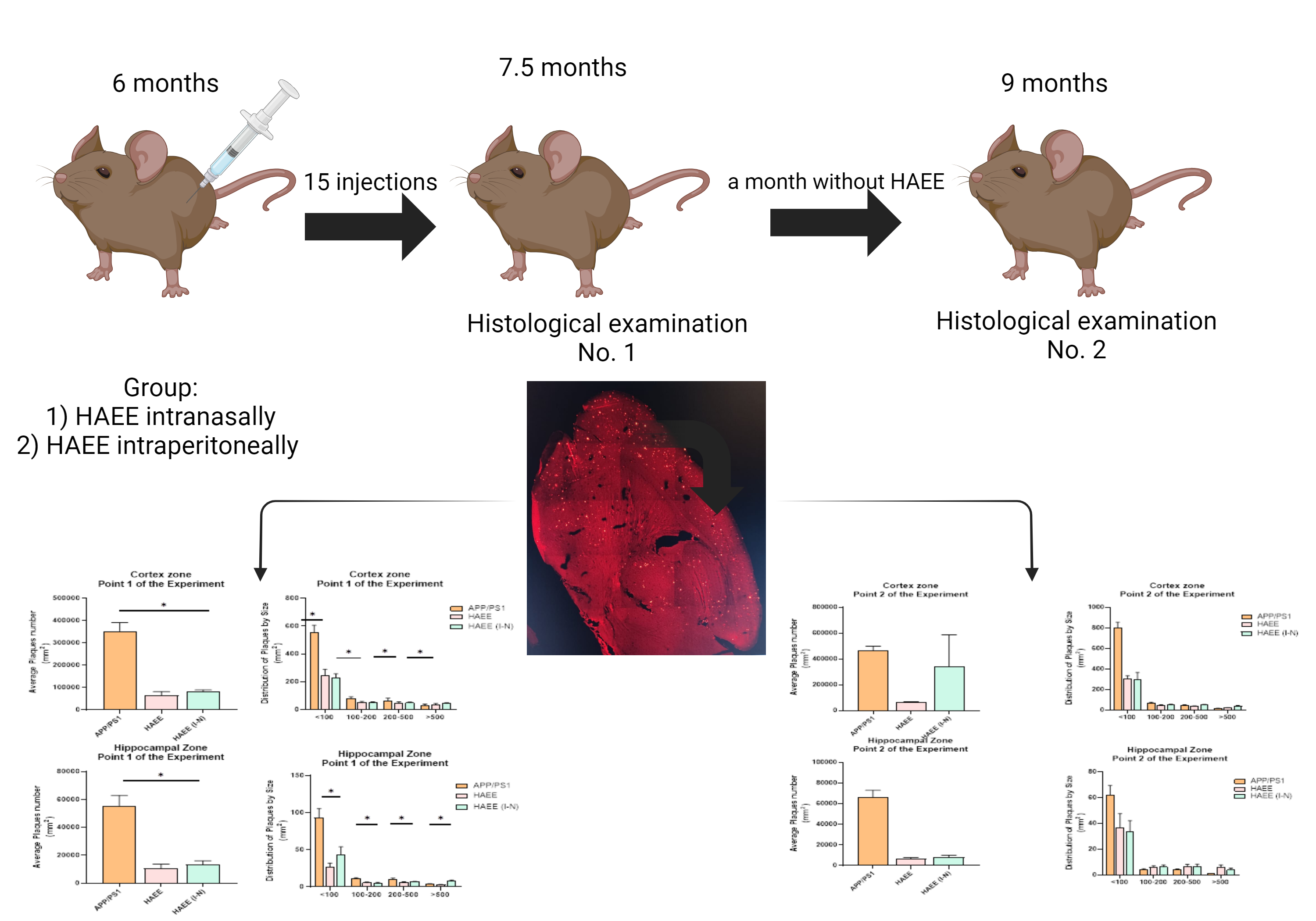
Материалы и методы: Исследование было проведено на 3 экспериментальных группах. В них использовались самцы мышей линии APPswe/PS1dE9/Blg на смешанном генетическом фоне с животными С57Bl6/Chg. Выборка мышей составила 10 единиц в каждой группе. В ходе исследование на 0 точке было произведено введение пептидов и препаратов 2 группам животных в возрасте 6 месяцев. Препараты вводились циркадно, через каждые 48 часов, без выходных, в течение одного месяца, далее на 1 точке эксперимента была отобрана половина группы (n=5) для дальнейшего гистологического анализа мозга мышей. Оставшаяся половина животных в течении месяца не получала препараты, затем было проведено гистологическое исследование мозга. Достоверность различий между экспериментальными группами и контрольной рассчитывали с использованием непарного критерия Стьюдента при р<0,05.
Результаты и обсуждения: Гистологическое исследование показало эффективность тетрапептида HAEE в дозировке 50 мг на 1 кг веса мыши, отмечается объективное снижение количества амилоидных бляшек в зоне коры головного мозга и гиппокампа мыши.
Заключение: Исследование показывает правильность предложенных гипотез и предполагает дальнейшее изучение и включение групп препаратов, рекомендованных при лечении болезни Альцгеймера и сравнение с ними.
Графическая аннотация
Ключевые слова:
тетрапептид, болезнь Альцгеймера, HAEE, лечение, APP/PSEN1Библиографические ссылки
Bach J, Haeusler D, Schmitt F (2020) The challenges of amyloid-targeting therapies in Alzheimer's disease: A critical appraisal. Journal of Alzheimer’s Disease 78(3): 921–935. https://doi.org/10.3233/JAD-200118
Froelich L, Haeusler D, Hoyer S (2019) Limitations of peptide-based treatments for Alzheimer's disease: An overview of the current status and future directions. Neuropharmacology 148: 146–157. https://doi.org/10.1016/j.neuropharm.2018.11.004
Korokin MV, Gudyrev OS, Pokrovskaya TG, Danilenko LM, Zhernakova NI, Avtina TV, Parshina AV, Pribylov SA, Lebedev PR, Kochkarov AA, Kuzubova EV, Radchenko AI, Koklin IS, Taran EI (2023) Features of bone remodeling and osteoreparation processes in modeling femoral fracture in genetically modified mice with impaired enzymatic regulation of steroid hormone metabolism. Research Results in Pharmacology 9(4): 113–123. https://doi.org/10.18413/rrpharmacology.9.10062
Lysikova EA, Kuzubova EV, Radchenko AI, Patrakhanov EA, Chaprov KD, Korokin MV, Deykin AV, Gudyrev OS, Pokrovskii MV (2023) The APPswe/PS1dE9/Blg transgenic mouse line for modeling cerebral amyloid angiopathy associated with Alzheimer’s disease. Molecular Biology 57(1): 74–82. https://doi.org/10.1134/S0026893323010077[PubMed]
Polikarpova AV, Egorova TV, Bardina MV (2022) Genetically modified animal models of hereditary diseases for testing of gene-directed therapy. Research Results in Pharmacology 8(2): 11–26. https://doi.org/10.3897/rrpharmacology.8.82618
Zhang Y, Wang Y, Li Z, Zhang L (2021) Limitations of current therapeutic strategies for Alzheimer’s disease: A review. Frontiers in Aging Neuroscience 13: 1–12. https://doi.org/10.3389/fnagi.2021.754123
Загрузки
Опубликован
Как цитировать
Выпуск
Раздел
Лицензия
Copyright (c) 2024 Patrakhanov EA

Это произведение доступно по лицензии Creative Commons «Attribution» («Атрибуция») 4.0 Всемирная.
 Русский
Русский
 English
English

