Study of the biotransformation of a new sydnonimine derivative with predominant cerebral vasodilatory activity
DOI:
https://doi.org/10.18413/rrpharmacology.11.1042Аннотация
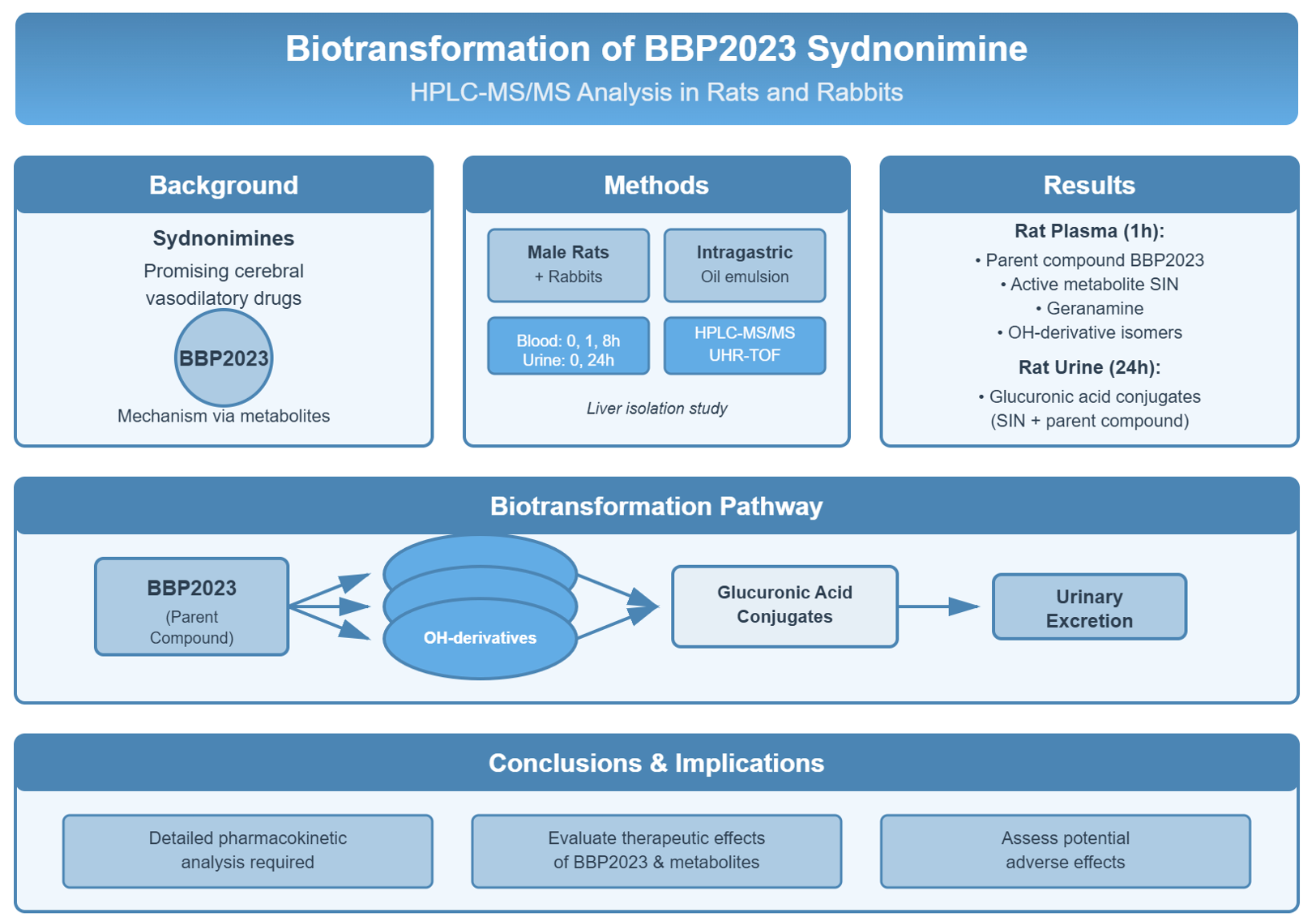
Introduction: Sydnonimines are a promising group of drugs for development of novel substances with predominant cerebral vasodilatory activity. One of them is experimental compound with laboratory code BBP20233. The mechanism of action of BBP2023 compound is tied to its metabolites. This study aims for identification of biotransformation products of BBP2023 using HPLC-MS/MS method.
Materials and Methods: Identification of BBP2023 biotransformation products was performed in male rats and male rabbits after intragastric administration of BBP2023 in form of oil emulsion. Blood samples were collected before and 1 and 8 hours after administration. Urine samples from rats were collected before and 24 hours after administration. Additionally, complete liver isolation was performed in another group of rats to investigate liver’s role in BBP2023 metabolism. Identification of products was performed using HPLC-MS/MS method as well as UHR-TOF method.
Results and Discussion: Analysis of rat plasma samples collected 1 hour after administration revealed the presence of parent compound, active metabolite SIN, geranamine and various isomers of OH-derivatives. Analysis of rat urine samples collected 24 hours after administration showed the presence of glucuronic acid conjugates of SIN and parent compound. Similar results were obtained from rabbit plasma samples, although with less variety of hydroxylated derivative isomers.
Conclusion: Investigated features of BBP2023 biotransformation require detailed analysis of pharmacokinetics for both BBP2023 and its metabolites to determine their role in therapeutic effect and possible adverse effects.
Графическая аннотация
Ключевые слова:
biotransformation, pharmacokinetics, sydnonimines, vasodilators, chromatography-mass spectrometryБиблиографические ссылки
Bohn H, Martorana PA, Schönafinger K (1992) Cardiovascular effects of the new nitric oxide donor, pirsidomine. Hemodynamic profile and tolerance studies in anesthetized and conscious dogs. European Journal of Pharmacology 220(1): 71–78. https://doi.org10.1016/0014-2999(92)90013-t [PubMed]
Dendorfer A (1996) Pharmacology of nitrates and other NO donors. Herz 1: 38–49. [in German] [PubMed]
Feelisch M, Ostrowski J, Noack E (1989) On the mechanism of NO release from sydnonimines. Journal of Cardiovascular Pharmacology 11: S13–22. [PubMed]
Fershtat LL, Zhilin ES (2021) Recent advances in the synthesis and biomedical applications of heterocyclic NO-donors. Molecules 26(18): 5705. https://doi.org10.3390/molecules26185705 [PubMed] [PMC]
Fumanal Idocin A, Specklin S, Taran F (2025) Sydnonimines: Synthesis, properties and applications in chemical biology. Chemical Communication 61(31): 5704–5718. https://doi.org10.1039/d5cc00535c [PubMed]
Furchgott RF, Zawadzki JV (1980) The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288(5789): 373–376. https://doi.org10.1038/288373a0 [PubMed]
Gaitatzis A, Sander JW (2013) The long-term safety of antiepileptic drugs. CNS Drugs 27(6): 435–455. https://doi.org10.1007/s40263-013-0063-0 [PubMed]
Granik VG, Grigoriev NB (2004) Nitric oxide (NO). A new way to find medicines. Vuzovskaya kniga, Moscow, 360 pp. [in Russian]
Ignarro LJ (1999) Nitric oxide: a unique endogenous signaling molecule in vascular biology. Bioscience Reports 19(2): 51–71. https://doi.org10.1023/a:1020150124721 [PubMed]
Janero DR, Ewing JF (2000) Nitric oxide and postangioplasty restenosis: pathological correlates and therapeutic potential. Free Radical Biology and Medicine 29(12): 1199–1221. https://doi.org10.1016/s0891-5849(00)00434-2 [PubMed]
Kim NJ, Baek JH, Lee J, Kim H, Song JK, Chun KH (2019) A PDE1 inhibitor reduces adipogenesis in mice via regulation of lipolysis and adipogenic cell signaling. Experimental and Molecular Medicine 51(1): 1–15. https://doi.org10.1038/s12276-018-0198-7 [PubMed] [PMC]
Liese A, Seelbach K, Wandrey Ch (2006) Industrial Biotransformations. Wiley‐VCH Verlag GmbH & Co. KGaA, Weinheim, 565 pp. https://doi.org/10.1002/3527608184
Popov NS, Balabanyan VYu, Kolgina NYu, Petrov GA, Donskov SA, Atadzhanov IB (2023) Quantitative determination of cyclic guanosine monoposphate (c-GMP) in rat tissues using liquid chromatography and tandem mass spectrometry. Pharmacokinetics and Pharmacodynamics (3): 28–38. https://doi.org/10.37489/2587-7836-2023-3-28-38 [in Russian]
Popov NS, Gavrilenko DA, Baranov MS, Balabanyan VYu (2024) Development of a method for quantitative determination of nitric oxide (NO) in rat tissues based on high-performance liquid chromatography and mass spectrometry. Pharmacy & Pharmacology 12(1): 49–62. https://doi.org/10.19163/2307-9266-2024-12-1-49-62
Rass V, Kindl P, Lindner A, Kofler M, Altmann K, Putnina L, Ianosi BA, Schiefecker AJ, Beer R, Pfausler B, Helbok R (2023) Blood pressure changes in association with nimodipine therapy in patients with spontaneous subarachnoid hemorrhage. Neurocritical Care 39(1): 104–115. https://doi.org10.1007/s12028-023-01760-y [PubMed] [PMC]
Rehse K, Schleifer KJ, Ciborski T, Bohn H (1993) New NO-donors with antithrombotic and vasodilating activities, II: 3-alkyl-N-nitroso-5-sydnone imines. Archiv der Pharmazie 326(10): 791–797. https://doi.org10.1002/ardp.19933261005 [PubMed]
Samarskaya AS, Nesmeyanov AN (2015) Organoelement derivatives of sydnonymines. PhD thesis, Moscow, Russia: Institute of Organoelement Compounds of the Russian Academy of Sciences. [in Russian]
Savolainen H (1982) Neurotoxicity of industrial chemicals and contaminants: Aspects of biochemical mechanisms and effects. In: Chambers CM, Chambers PL (Eds) New Toxicology for Old. Archives of Toxicology, Vol 5. Springer, Berlin, Heidelberg, 71–83. https://doi.org/10.1007/978-3-642-68511-8_12
Schrammel A, Pfeiffer S, Schmidt K, Koesling D, Mayer B (1998) Activation of soluble guanylyl cyclase by the nitrovasodilator 3-morpholinosydnonimine involves formation of S-nitrosoglutathione. Molecular Pharmacology 54(1): 207–212. https://doi.org10.1124/mol.54.1.207 [PubMed]
Zetterström R (2009) The 1998 Nobel Prize-discovery of the role of nitric oxide as a signalling molecule. Acta Paediatrica 98(3): 593–599. https://doi.org10.1111/j.1651-2227.2008.01121.x [PubMed]
Загрузки
Опубликован
Как цитировать
Выпуск
Раздел
Лицензия
Copyright (c) 2025 Popov NS, Gavrilenko DA, Pavlov RD, Baranov MS, Kaurova DE, Myasnyanko IN, Ivanov DS, Balabanyan VYu

Это произведение доступно по лицензии Creative Commons «Attribution» («Атрибуция») 4.0 Всемирная.
 Русский
Русский
 English
English

