Orthogonal approach and critical quality attributes for gene and cell therapy products
DOI:
https://doi.org/10.18413/rrpharmacology.11.738Аннотация
Introduction: Gene and cell therapy (GCT) products are revolutionizing medicine because they are made up of unique biological components like genetic material, viral vectors, and viable cells. However, their complexity necessitates rigorous quality control strategies to ensure efficacy, safety, and batch consistency. This manuscript explores the application of an orthogonal approach – employing multiple independent methods – to assess critical quality attributes, such as identity, potency, and purity of GCT products.
Materials and Methods: To achieve the aim of our work, we analyzed 15 GCT products for 11 different types of diseases and reports of multiple regulatory agencies.
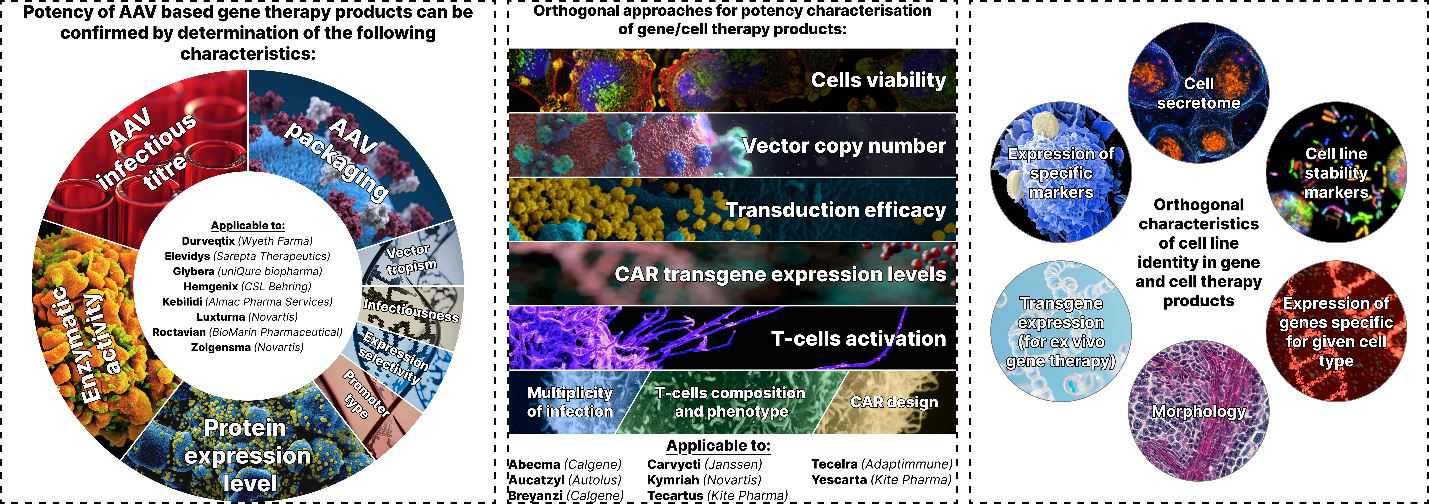
Results: For cell-based therapies, identity is confirmed through genotypic, phenotypic, and morphological analyses, while potency is evaluated using functional assays tailored to the product’s mechanism of action, such as cell viability, differentiation status, or cytokine secretion. Viral vector-based therapies require characterization of structural integrity, transgene expression, and the ratio of full to empty capsids, employing techniques like dynamic light scattering (DLS), PCR, and ELISA.
Conclusion: The paper highlights regulatory recommendations from the FDA, EMA, and WHO, emphasizing the need for validated assays during product release and stability testing. Case studies, including CAR-T cells and AAV-based therapies, illustrate the practical implementation of orthogonal methods. Challenges such as assay variability and the need for clinical correlation are discussed, underscoring the importance of assay development early in the product lifecycle. By integrating diverse analytical techniques, the orthogonal approach ensures comprehensive product characterization facilitating the translation of GCTs from research to clinical application.
Графическая аннотация
Ключевые слова:
orthogonal approach, quality attributes, quality control, cell therapy, gene therapyБиблиографические ссылки
Ahmed I, Johnston Jr RJ, Singh MS (2021) Pluripotent stem cell therapy for retinal diseases. Annals of Translational Medicine 9(15): 1279–1279. https://doi.org/10.21037/atm-20-4747[PubMed]
Bartz C, Meixner M, Giesemann P, Roël G, Bulwin G-C, Smink JJ (2016) An ex vivo human cartilage repair model to evaluate the potency of a cartilage cell transplant. Journal of Translational Medicine 14: 317. https://doi.org/10.1186/s12967-016-1065-8 [PubMed] [PMC]
Buck T, Wijnholds J (2020) Recombinant adeno-associated viral vectors (rAAV)-vector elements in ocular gene therapy clinical trials and transgene expression and bioactivity assays. International Journal of Molecular Sciences 21: 4197. https://doi.org/10.3390/ijms21124197 [PubMed] [PMC]
Center for Biologics Evaluation and Research (CBER) (2020) Chemistry, Manufacturing, and Control (CMC) Information for Human Gene Therapy Investigational New Drug Applications (INDs). Guidance for Industry. Final (2008-D-0205). https://www.fda.gov/media/113760/download
Center for Biologics Evaluation and Research (CBER) (2024a) Considerations for the Development of Chimeric Antigen Receptor (CAR) T Cell Products. Guidance document. Final (FDA-2021-D-0404). https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-development-chimeric-antigen-receptor-car-t-cell-products
Center for Biologics Evaluation and Research (CBER) (2024b) Human Gene Therapy Products Incorporating Human Genome Editing. Guidance for Industry. Final (FDA-2021-D-0398). https://www.fda.gov/regulatory-information/search-fda-guidance-documents/human-gene-therapy-products-incorporating-human-genome-editing
CHMP, CAT (2020) Zolgensma. Assessment report (EMA/200482/2020). https://www.ema.europa.eu/en/documents/assessment-report/zolgensma-epar-public-assessment-report_en.pdf
Cole L, Fernandes D, Hussain MT, Kaszuba M, Stenson J, Markova N (2021) Characterization of recombinant adeno-associated viruses (rAAVs) for gene therapy using orthogonal techniques. Pharmaceutics 13(4): 586. https://doi.org/10.3390/pharmaceutics13040586[PubMed] [PMC]
Committee for Advanced Therapies (CAT) (2011) Reflection paper on stem cell-based medicinal products (EMA/CAT/571134/2009). https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-stem-cell-based-medicinal-products_en.pdf
Committee for Advanced Therapies (CAT) (2018) Guideline on the Quality, Non-clinical and Clinical Aspects of Gene Therapy Medicinal Products (EMA/CAT/80183/2014). https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-non-clinical-and-clinical-aspects-gene-therapy-medicinal-products_en.pdf
Committee for Advanced Therapies (CAT) (2020) Guideline on Quality, Non-clinical and Clinical Aspects of Medicinal Products Containing Genetically Modified Cells (EMA/CAT/GTWP/671639/2008 Rev. 1 - corr). https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-non-clinical-and-clinical-aspects-medicinal-products-containing-genetically-modified-cells-revision-1_en.pdf
Committee for Advanced Therapies (CAT) (2022) Kymriah. Assessment report (EMEA/H/C/004090/P46/017). https://www.ema.europa.eu/en/documents/variation-report/kymriah-h-c-4090-p46-017-epar-assessment-report_en.pdf
Committee for Advanced Therapies (CAT) (2024) Guideline on Quality, Non-clinical and Clinical Requirements for Investigational Advanced Therapy Medicinal Products in Clinical Trials. Guidance document. Draft (EMA/CAT/123573/2024). https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-quality-non-clinical-clinical-requirements-investigational-advanced-therapy-medicinal-products-clinical-trials-second-version_en.pdf
Committee for Medicinal Products for Human Use (CHMP) (2008) Guideline on Human Cell-Based Medicinal Products (EMEA/CHMP/410869/2006). https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-human-cell-based-medicinal-products_en.pdf
Committee for Medicinal Products for Human Use (CHMP) (2016a) Guideline on Potency Testing of Cell Based Immunotherapy Medicinal Products for the Treatment of Cancer (EMA/CHMP/BWP/271475/2006 rev.1). https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-potency-testing-cell-based-immunotherapy-medicinal-products-treatment-cancer-revision-1_en.pdf
Committee for Medicinal Products for Human Use (CHMP) (2016b) Strimvelis. Assessment report (EMA/CHMP/272303/2016 Rev 1.). https://www.ema.europa.eu/en/documents/assessment-report/strimvelis-epar-public-assessment-report_en.pdf
Committee for Medicinal Products for Human Use (CHMP) (2017) Alofisel. Assessment report (EMA/CHMP/64055/2018 Corr.). https://www.ema.europa.eu/en/documents/assessment-report/alofisel-epar-public-assessment-report_en.pdf
Committee for Medicinal Products for Human Use (CHMP) (2018a) Durveqtix. Assessment report (EMA/323453/2024). https://www.ema.europa.eu/en/medicines/human/EPAR/beqvez
Committee for Medicinal Products for Human Use (CHMP) (2018b) Luxturna. Assessment report (EMA/CHMP/700911/2018). https://www.ema.europa.eu/en/documents/assessment-report/luxturna-epar-public-assessment-report_en.pdf
Committee for Medicinal Products for Human Use (CHMP) (2018c) Yescarta. Assessment report (EMA/481168/2018). https://www.ema.europa.eu/en/documents/assessment-report/yescarta-epar-public-assessment-report_en.pdf
Committee for Medicinal Products for Human Use (CHMP) (2019) Zynteglo. Assessment report (EMA/56140/2020/Corr.1). https://www.ema.europa.eu/en/documents/assessment-report/zynteglo-epar-public-assessment-report_en.pdf
Committee for Medicinal Products for Human Use (CHMP) (2020) Libmeldy. CHMP assessment report (EMA/584450/2020). https://www.ema.europa.eu/en/documents/assessment-report/libmeldy-epar-public-assessment-report_en.pdf
Committee for Medicinal Products for Human Use (CHMP) (2022a) Roctavian. Assessment report (EMA/685615/2022). https://www.ema.europa.eu/en/documents/assessment-report/roctavian-epar-public-assessment-report_en.pdf
Committee for Medicinal Products for Human Use (CHMP) (2022b) Upstaza. Assessment report (EMA/CHMP/571076/2022). https://www.ema.europa.eu/en/documents/assessment-report/upstaza-epar-public-assessment-report_en.pdf
Couto LB, Buchlis G, Farjo R, High K (2016) Potency assay for AAV vector encoding retinal pigment epithelial 65 protein. In: The Association for Research in Vision and Ophthalmology. Investigative Ophthalmology & Visual Science, Seattle, 759. https://iovs.arvojournals.org/article.aspx?articleid=2559539
Cristi F, Gutiérrez T, Hitt MM, Shmulevitz M (2022) Genetic modifications that expand oncolytic virus potency. Frontiers in Molecular Biosciences 9: 831091. https://doi.org/10.3389/fmolb.2022.831091 [PubMed] [PMC]
Da Silva Meirelles L, Fontes AM, Covas DT, Caplan AI (2009) Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine & Growth Factor Reviews 20(5-6): 419–427. https://doi.org/10.1016/j.cytogfr.2009.10.002 [PubMed]
De Wolf C, Van De Bovenkamp M, Hoefnagel M (2018) Regulatory perspective on in vitro potency assays for human dendritic cells used in anti-tumor immunotherapy. Cytotherapy 20(11): 1289–1308. https://doi.org/10.1016/j.jcyt.2018.07.006 [PubMed]
Debauve G (2024) Minimizing the impact of stability studies on gene therapy batch yield. USP Stakeholder Forum. https://www.usp.org/sites/default/files/usp/document/events-and-training/03-minimizing-the-impact-of-stability-studies-on-gene-therapy-batch-yield.pdf
European Medicines Agency (EMA) (2016) Glybera. EPAR summary for the public (EMA/670094/2015). https://www.ema.europa.eu/en/documents/overview/glybera-epar-summary-public_en.pdf
European Medicines Agency (EMA) (2021) Spherox. Summary for the public (EMA/326451/2021). https://www.ema.europa.eu/en/documents/overview/spherox-epar-summary-public_en.pdf
European Pharmacopoeia (2025) 5.3 Statistical analysis of results of biological assays and tests. https://pheur.edqm.eu/home
Ferrucci PF, Pala L, Conforti F, Cocorocchio E (2021) Talimogene laherparepvec (T-VEC): an intralesional cancer immunotherapy for advanced melanoma. Cancers 13(6): 1383. https://doi.org/10.3390/cancers13061383 [PubMed] [PMC]
Fucikova J, Palova-Jelinkova L, Bartunkova J, Spisek R (2019) Induction of tolerance and immunity by dendritic cells: Mechanisms and clinical applications. Frontiers in Immunology 10: 2393. https://doi.org/10.3389/fimmu.2019.02393 [PubMed] [PMC]
Guillén-García P, Guillén-Vicente I, Rodríguez-Iñigo E, Guillén-Vicente M, Fernández-Jaén TF, Navarro R, Aboli L, Torres R, Abelow S, López-Alcorocho JM (2023) Cartilage defect treatment using high-density autologous chondrocyte implantation (HD-ACI). Bioengineering 10(9): 1083. https://doi.org/10.3390/bioengineering10091083 [PubMed] [PMC]
Li C, Samulski RJ (2020) Engineering adeno-associated virus vectors for gene therapy. Nature Reviews Genetics 21(4): 255–272. https://doi.org/10.1038/s41576-019-0205-4[PubMed]
Mazinani M, Rahbarizadeh F (2022) CAR-T cell potency: from structural elements to vector backbone components. Biomarker Research 10(1): 70. https://doi.org/10.1186/s40364-022-00417-w [PubMed] [PMC]
Melnikova EV, Rachinskaya OA, Merkulov VA (2021) Advanced therapy medicines based on oncolytic viruses (Part I: Development and authorisation of products in China). The Bulletin of the Scientific Centre for Expert Evaluation of Medicinal Products [Vedomosti Nauchnogo Tsentra Ekspertizy Sredstv Meditsinskogo Primeneniy] 11(3): 148–159. https://doi.org/10.30895/1991-2919-2021-11-148-159 [in Russian]
Melnikova EV, Merkulov VA, Merkulova OV (2023) Gene therapy of neurodegenerative diseases: achievements, developments, and clinical implementation challenges. Biological Products. Prevention, Diagnosis, Treatment [BIOpreparaty. Profilaktika, Diagnostika, Lechenie] 23(2): 127–147. https://doi.org/10.30895/2221-996X-2023-433 [in Russian]
Melnikova EV, Rachinskaya OA, Trusov GA, Khorolsky MD, Semenova IS, Tereshkina NV, Merkulov VA (2019) Justification of methodological approaches to identification testing of biomedical cell products. Biological Products. Prevention, Diagnosis, Treatment [BIOpreparaty. Profilaktika, Diagnostika, Lechenie] 19(1): 28–38. https://doi.org/10.30895/2221-996X-2019-19-1-28-38 [in Russian]
Ministry of Health of the Russian Federation [Ministerstvo Zdravookhraneniia Rossiiskoi Federatsii] (2017) Order N 14n of 19 January 2017 “On Approving the Specification Form for the Biomedical Cell Product” [Prikaz ot 19 janvarja 2017 goda N 14n “Ob utverzhdenii formy spetsifikatsii na biomeditsinskii kletochnyy product”] [in Russian].
Ministry of Food and Drug Safety, Republic of Korea (MFDS) (2018) Guideline on Quality Assessment for Gene-Editing Based Advanced Therapy Medicinal Products. https://mfds.go.kr/eng/brd/m_27/down.do?brd_id=eng0005&seq=71877&data_tp=A&file_seq=1
Narayan VM, Meeks JJ, Jakobsen JS, Shore ND, Sant GR, Konety BR (2024) Mechanism of action of nadofaragene firadenovec-vncg. Frontiers in Oncology 14: 1359725. https://doi.org/10.3389/fonc.2024.1359725 [PubMed] [PMC]
Pharmeuropa 34.3 (2022) General Monograph. Gene Therapy Medicinal Products for Human Use (3186). European Pharmacopoeia. https://pheur.edqm.eu/home
Pokrovsky NS, Vodyakova MA, Merkulov VA, Melnikova EV (2024) Specific aspects and key parameters of validation for methods of phenotyping of cell lines included in cell therapy products. Immunologiya [Immunologiya] 45(3): 343–354. https://doi.org/10.33029/1816-2134-2024-45-3-343-354 [in Russian]
Salmikangas P, Carlsson B, Klumb C, Reimer T, Thirstrup S (2023) Potency testing of cell and gene therapy products. Frontiers in Medicine 10: 1190016. https://doi.org/10.3389/fmed.2023.1190016 [PubMed] [PMC]
Schofield M (2021) Empty/full separation: gene therapy’s hidden challenge. Cell and Gene Therapy Insights 6: 1715–1722. https://doi.org/10.18609/cgti.2020.189
Council of the Eurasian Economic Commission [Sovet Evraziiskoi Ekonomicheskoi Komissii] (2016) Resolution dated November 3, 2016, No. 89 “On Adoption of Rules of Conducting Trials of Biological Medications of the Eurasian Economic Union”. https://docs.cntd.ru/document/456026116 [in Russian].
The American National Standards Institute oversees standards and conformity assessment activities in the United States (2022) ANSI/ATCC ASN-0002-2022. Authentication Of Human Cell Lines: Standardization Of Short Tandem Repeat (STR) Profiling – Revised 2022. https://webstore.ansi.org/standards/atcc/ansiatccasn00022022?srsltid=AfmBOorFg16EGVRrUILmQ8adJ3IV7BDqdaZ4Vp5VWThxCce1RWMbUfll
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) (1995) ICH Topic Q2 (R1) Validation of Analytical Procedures: Text and Methodology. ICH Harmonised Tripartite Guideline (CPMP/ICH/381/95). https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q2r1-validation-analytical-procedures-text-and-methodology-step-5-first-version_en.pdf
The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) (1998) ICH Q5D Derivation and characterisation of cell substrates used for production of biotechnological/biological products (CPMP/ICH/294/95). https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-5-d-derivation-and-characterisation-cell-substrates-used-production-biotechnologicalbiological-products-step-5_en.pdf
The National Institute of Health Sciences (NIHS). Division of Medical Devices (DMD) (2013) Guidance on Evaluation of Autologous Induced Pluripotent Stem Cells-derived Retinal Pigment Epithelial Cells (PFSB/ELD/OMDE (Yakushokuki) Notification 0529 No.1). https://dmd.nihs.go.jp/jisedai/tsuuchi/auto_iPS_derived_retinal.pdf
The United States Food and Drug Administration (FDA) (2011) Potency Tests for Cellular and Gene Therapy Products. Guidance for Industry. Final (FDA-2008-D-0520). https://www.fda.gov/media/79856/download
The United States Food and Drug Administration (FDA) (2022) ADSTILADRIN. Summary Basis for Regulatory Action (125700/0). https://www.fda.gov/media/164532/download?attachment
The United States Pharmacopeia (USP) (2024) Innovative Analytical Approaches to Cell and Gene Therapy. Executive Summary. https://www.usp.org/sites/default/files/usp/document/events-training/USP-Bio-SF_Exec%20summary_postSMRP-072424.pdf
The United States Pharmacopoeia (2024a) 〈1034〉 Analysis of Biological Assays. https://doi.org/10.31003/USPNF_M5677_02_01
The United States Pharmacopoeia (2024b) 〈1047〉Gene Therapy Products. https://doi.org/10.31003/USPNF_M3024_02_01
The United States Pharmacopoeia (2024c) US Pharmacopeia (USP). https://www.usp.org/
Trento C, Bernardo ME, Nagler A, Kuçi S, Bornhäuser M, Köhl U, Strunk D, Galleu A, Sanchez-Guijo F, Gaipa G, Introna M, Bukauskas A, Le Blanc K, Apperley J, Roelofs H, Van Campenhout A, Beguin Y, Kuball J, Lazzari L, Avanzini MA, Fibbe W, Chabannon C, Bonini C, Dazzi F (2018) Manufacturing mesenchymal stromal cells for the treatment of graft-versus-host disease: A survey among centers affiliated with the european society for blood and marrow transplantation. Biology of Blood and Marrow Transplantation 24(11): 2365–2370. https://doi.org/10.1016/j.bbmt.2018.07.015 [PubMed] [PMC]
Wagner AK, Alici E, Lowdell MW (2019) Characterization of human natural killer cells for therapeutic use. Cytotherapy 21(3): 315–326. https://doi.org/10.1016/j.jcyt.2018.11.001 [PubMed]
Weiss ARR, Dahlke MH (2019) Immunomodulation by mesenchymal stem cells (MSCs): Mechanisms of action of living, apoptotic, and dead MSCs. Frontiers in Immunology 10: 1191. https://doi.org/10.3389/fimmu.2019.01191 [PubMed] [PMC]
World Health Organization (WHO) (2013) Recommendations for the evaluation of animal cell cultures as substrates for the manufacture of biological medicinal products and for the characterization of cell banks, Annex 3. Technical Report Series (978). https://cdn.who.int/media/docs/default-source/biologicals/documents/trs_978_annex_3.pdf?sfvrsn=fe61af77_3&download=true
Zha K, Li X, Yang Z, Tian G, Sun Z, Sui X, Dai Y, Liu S, Guo Q (2021) Heterogeneity of mesenchymal stem cells in cartilage regeneration: from characterization to application. NPJ Regenerative Medicine 6(1): 14. https://doi.org/10.1038/s41536-021-00122-6[PubMed] [PMC]
Zhang W-W, Li L, Li D, Liu J, Li X, Li W, Xu X, Zhang MJ, Chandler LA, Lin H, Hu A, Xu W, Lam DM-K (2018) The first approved gene therapy product for cancer Ad- p53 (Gendicine): 12 years in the clinic. Human Gene Therapy 29(2): 160–179. https://doi.org/10.1089/hum.2017.218 [PubMed]
Загрузки
Опубликован
Как цитировать
Выпуск
Раздел
Лицензия
Copyright (c) 2025 Melnikova EV, Vodyakova MA, Pokrovsky NS, Merkulov VA

Это произведение доступно по лицензии Creative Commons «Attribution» («Атрибуция») 4.0 Всемирная.
 Русский
Русский
 English
English

