Semaglutide reduces hypothalamic glial cell damage in a streptozocin-induced model of Alzheimer's disease
DOI:
https://doi.org/10.18413/rrpharmacology.11.804Аннотация
Introduction: Reactive changes in glial cells, as well as their dysfunction, particularly cerebrospinal fluid dynamics disturbances, are associated with multiple neurodegenerative diseases and Alzheimer’s disease (AD). Intraventricular administration of streptozotocin (STZ) is considered a model of sporadic AD, though data on hypothalamic glial cell changes, ependymal glia, and tanycytes in this model remain limited. Of particular interest is the potential for glucagon-like peptide-1 (GLP-1) receptor agonists to address STZ-induced changes following intraventricular administration.
Material and Methods: Using immunomorphological methods, this study assessed changes in staining density and distribution of glial proteins (GFAP, aquaporine-4, connexin 43, vimentin) and neuronal alterations in hypothalamic structures following intraventricular STZ administration (3 mg/kg) and course treatment with intraperitoneal semaglutide (0.1 mg/kg, 16 injections).
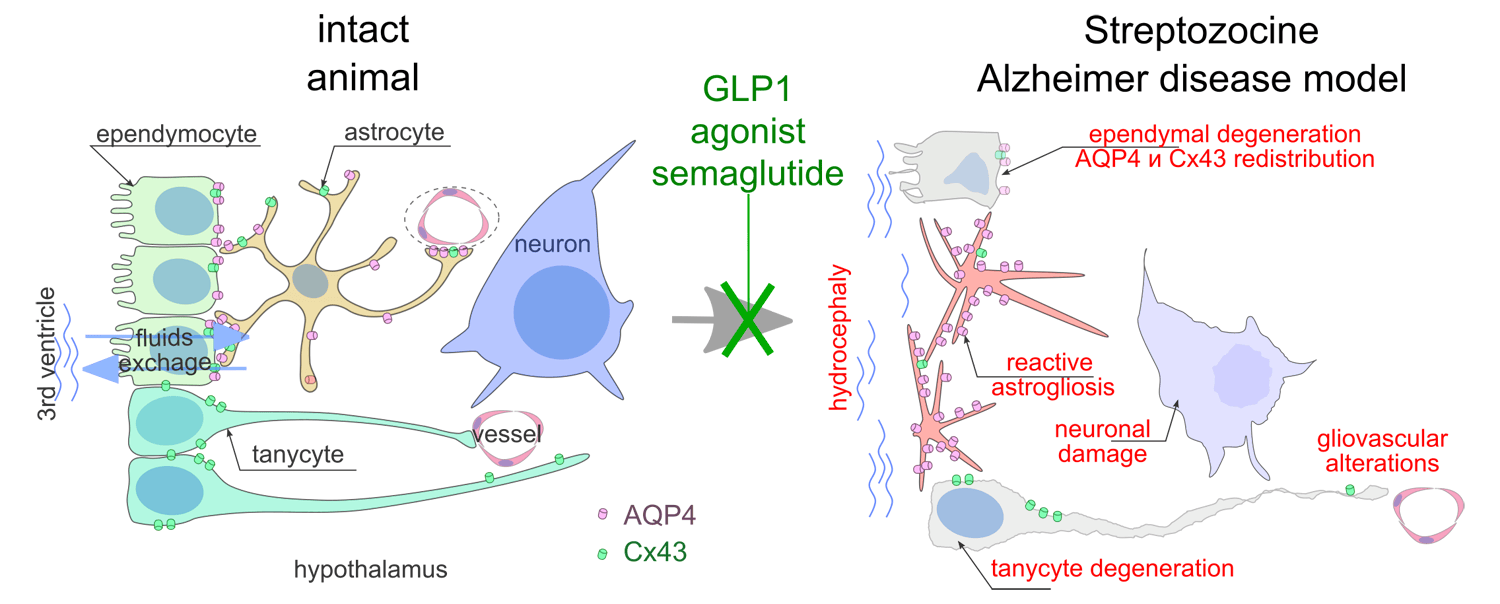
Results: STZ caused neuronal damage in the ventromedial hypothalamic nucleus, disrupted the ependymal lining and tanycytes of the third ventricle, induced reactive astrogliosis, and altered the distribution of aquaporin-4 and connexin-43. Semaglutide administration reduced astroglial activation, normalized aquaporin and connexin distribution, decreased neuronal death, and suppressed caspase-3 activation in the ventromedial hypothalamic nucleus.
Conclusion: Intraventricular single-dose STZ administration causes long-term impairment of glial functions related to cerebrospinal fluid exchange. The course treatment GLP-1R agonist semaglutide (started 5 days after streptozocin administration, 16 injections every other day) demonstrated normalizing effects on both glial and neuronal parameters in the STZ-induced AD model.
Графическая аннотация
Ключевые слова:
astrocytes, ependymal cells, Alzheimer's disease, streptozotocin, semaglutideБиблиографические ссылки
Aal- Agrawal R, Tyagi E, Shukla R, Nath C (2011) Insulin receptor signaling in rat hippocampus: A study in STZ (ICV) induced memory deficit model. European Neuropsychopharmacology 21(3): 261–273. https://doi.org/10.1016/j.euroneuro.2010.11.009 [PubMed]
Aras R, Barron AM, Pike CJ (2012) Caspase activation contributes to astrogliosis. Brain Research 1450: 102–115. https://doi.org/10.1016/j.brainres.2012.02.056 [PubMed] [PMC]
Biasibetti R, Almeida Dos Santos JP, Rodrigues L, Wartchow KM, Suardi LZ, Nardin P, Selistre NG, Vázquez D, Gonçalves C-A (2017) Hippocampal changes in STZ-model of Alzheimer’s disease are dependent on sex. Behavioural Brain Research 316: 205–214. https://doi.org/10.1016/j.bbr.2016.08.057 [PubMed]
Chen Y, Liang Z, Blanchard J, Dai C-L, Sun S, Lee MH, Grundke-Iqbal I, Iqbal K, Liu F, Gong C-X (2013) A non-transgenic mouse model (icv-STZ mouse) of Alzheimer’s disease: similarities to and differences from the transgenic model (3xTg-AD mouse). Molecular Neurobiology 47(2): 711–725. https://doi.org/10.1007/s12035-012-8375-5 [PubMed] [PMC]
Cibelli A, Stout R, Timmermann A, De Menezes L, Guo P, Maass K, Seifert G, Steinhäuser C, Spray DC, Scemes E (2021) Cx43 carboxyl terminal domain determines AQP4 and Cx30 endfoot organization and blood brain barrier permeability. Scientific Reports 11(1): 24334.https://doi.org/10.1038/s41598-021-03694-x [PubMed] [PMC]
Cummings JL, Atri A, Feldman HH, Hansson O, Sano M, Knop FK, Johannsen P, León T, Scheltens P (2025) evoke and evoke+: design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating efficacy, safety, and tolerability of semaglutide in early-stage symptomatic Alzheimer’s disease. Alzheimer’s Research & Therapy 17(1): 14. https://doi.org/10.1186/s13195-024-01666-7 [PubMed] [PMC]
Dragić M, Zarić M, Mitrović N, Nedeljković N, Grković I (2019) Application of gray level co-occurrence matrix analysis as a new method for enzyme histochemistry quantification. Microscopy and Microanalysis 25(3): 690–698. https://doi.org/10.1017/s1431927618016306[PubMed]
Duquenne M, Deligia E, Folgueira C, Bourouh C, Caron E, Pfrieger F, Schwaninger M, Nogueiras R, Annicotte J-S, Imbernon M, Prévot V (2024) Tanycytic transcytosis inhibition disrupts energy balance, glucose homeostasis and cognitive function in male mice. Molecular Metabolism 87: 101996. https://doi.org/10.1016/j.molmet.2024.101996 [PubMed] [PMC]
Estato V, Obadia N, Chateaubriand PH, Figueiredo V, Curty M, Costa Silva M, Ferreira RGL, Santa-Ritta J, Campos Baroni M, Aragão A, Neno JOG, Vasconcellos CAM, Costa D’Avila J, Gomes Granja M, Caire De Castro Faria-Neto H (2025) Semaglutide restores astrocyte-vascular interactions and blood-brain barrier integrity in a model of diet-induced metabolic syndrome. Diabetology & Metabolic Syndrome 17(1): 2. https://doi.org/10.1186/s13098-024-01528-0 [PubMed] [PMC]
Fabian‐Fine R, Weaver AL, Roman AG, Winters MJ, DeWitt JC (2024) Myelinated glial cells: Their proposed role in waste clearance and neurodegeneration in arachnid and human brain. Journal of Comparative Neurology 532(11): e70000. https://doi.org/10.1002/cne.70000[PubMed] [PMC]
Fan M, Liu S, Sun H-M, Ma M-D, Gao Y-J, Qi C-C, Xia Q-R, Ge J-F (2022) Bilateral intracerebroventricular injection of streptozotocin induces AD-like behavioral impairments and neuropathological features in mice: Involved with the fundamental role of neuroinflammation. Biomedicine & Pharmacotherapy 153: 113375. https://doi.org/10.1016/j.biopha.2022.113375 [PubMed]
Fernandez AM, Martinez-Rachadell L, Navarrete M, Pose-Utrilla J, Davila JC, Pignatelli J, Diaz-Pacheco S, Guerra-Cantera S, Viedma-Moreno E, Palenzuela R, Ruiz De Martin Esteban S, Mostany R, Garcia-Caceres C, Tschöp M, Iglesias T, De Ceballos ML, Gutierrez A, Torres Aleman I (2022) Insulin regulates neurovascular coupling through astrocytes. Proceedings of the National Academy of Sciences 119(29): e2204527119. https://doi.org/10.1073/pnas.2204527119 [PubMed] [PMC]
Gayger-Dias V, Menezes L, Da Silva V-F, Stiborski A, Silva ACR, Sobottka TM, Quines-Silva VC, Pakulski-Souto B, Bobermin LD, Quincozes-Santos A, Leite MC, Gonçalves C-A (2024) Changes in astroglial water flow in the pre-amyloid phase of the STZ model of AD dementia.Neurochemical Research 49(7): 1851–1862. https://doi.org/10.1007/s11064-024-04144-6[PubMed]
Grech O, Mitchell JL, Lyons HS, Yiangou A, Thaller M, Tsermoulas G, Brock K, Mollan SP, Sinclair AJ (2024) Effect of glucagon like peptide-1 receptor agonist exenatide, used as an intracranial pressure lowering agent, on cognition in Idiopathic Intracranial Hypertension. Eye 38(7): 1374–1379. https://doi.org/10.1038/s41433-023-02908-y [PubMed] [PMC]
Grieb P (2016) Intracerebroventricular streptozotocin injections as a model of Alzheimer’s disease: In search of a relevant mechanism. Molecular Neurobiology 53(3): 1741–1752. https://doi.org/10.1007/s12035-015-9132-3 [PubMed] [PMC]
Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, Singh I, Deane R, Nedergaard M (2014) Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. The Journal of Neuroscience 34(49): 16180–16193. https://doi.org/10.1523/jneurosci.3020-14.2014 [PubMed] [PMC]
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Science Translational Medicine 4(147): 147ra111. https://doi.org/10.1126/scitranslmed.3003748 [PubMed] [PMC]
Kamat PK, Kalani A, Rai S, Tota SK, Kumar A, Ahmad AS (2016) Streptozotocin intracerebroventricular-induced neurotoxicity and brain insulin resistance: a therapeutic intervention for treatment of sporadic Alzheimer’s disease (sAD)-like pathology. Molecular Neurobiology 53(7): 4548–4562. https://doi.org/10.1007/s12035-015-9384-y [PubMed]
Kelly P, McClean PL, Ackermann M, Konerding MA, Hölscher C, Mitchell CA (2015) Restoration of cerebral and systemic microvascular architecture in APP/PS1 transgenic Mice following treatment with LiraglutideTM. Microcirculation 22(2): 133–145. https://doi.org/10.1111/micc.12186 [PubMed]
Li Y, Xu P, Shan J, Sun W, Ji X, Chi T, Liu P, Zou L (2020) Interaction between hyperphosphorylated tau and pyroptosis in forskolin and streptozotocin induced AD models. Biomedicine & Pharmacotherapy 121: 109618. https://doi.org/10.1016/j.biopha.2019.109618 [PubMed]
Liang T, Chang F, Huang Z, Peng D, Zhou X, Liu W (2023) Evaluation of glymphatic system activity by diffusion tensor image analysis along the perivascular space (DTI-ALPS) in dementia patients. The British Journal of Radiology 96(1146): 20220315. https://doi.org/10.1259/bjr.20220315 [PubMed] [PMC]
Masai K, Nakayama Y, Shin K, Sugahara C, Miyazaki I, Yasuhara T, Date I, Asanuma M (2024) Neurogenesis impairment with glial activation in the hippocampus-connected regions of intracerebroventricular streptozotocin-injected mice. Neuroscience Letters 820: 137598. https://doi.org/10.1016/j.neulet.2023.137598 [PubMed]
Park J-S, Kam T-I, Lee S, Park H, Oh Y, Kwon S-H, Song J-J, Kim D, Kim H, Jhaldiyal A, Na DH, Lee KC, Park EJ, Pomper MG, Pletnikova O, Troncoso JC, Ko HS, Dawson VL, Dawson TM, Lee S (2021) Blocking microglial activation of reactive astrocytes is neuroprotective in models of Alzheimer’s disease. Acta Neuropathologica Communications 9(1): 78. https://doi.org/10.1186/s40478-021-01180-z [PubMed] [PMC]
Popov A, Brazhe A, Denisov P, Sutyagina O, Li L, Lazareva N, Verkhratsky A, Semyanov A (2021) Astrocyte dystrophy in ageing brain parallels impaired synaptic plasticity. Aging Cell 20(3): e13334. https://doi.org/10.1111/acel.13334 [PubMed] [PMC]
Qi G, Tang H, Hu J, Kang S, Qin S (2025) Potential role of tanycyte-derived neurogenesis in Alzheimer’s disease. Neural Regeneration Research 20(6): 1599–1612. https://doi.org/10.4103/nrr.nrr-d-23-01865 [PubMed] [PMC]
Raikwar SP, Bhagavan SM, Ramaswamy SB, Thangavel R, Dubova I, Selvakumar GP, Ahmed ME, Kempuraj D, Zaheer S, Iyer S, Zaheer A (2019) Are tanycytes the missing link between type 2 diabetes and Alzheimer’s disease? Molecular Neurobiology 56(2): 833–843. https://doi.org/10.1007/s12035-018-1123-8 [PubMed] [PMC]
Roales-Buján R, Páez P, Guerra M, Rodríguez S, Vío K, Ho-Plagaro A, García-Bonilla M, Rodríguez-Pérez L-M, Domínguez-Pinos M-D, Rodríguez E-M, Pérez-Fígares J-M, Jiménez A-J (2012) Astrocytes acquire morphological and functional characteristics of ependymal cells following disruption of ependyma in hydrocephalus. Acta Neuropathologica 124(4): 531–546. https://doi.org/10.1007/s00401-012-0992-6 [PubMed] [PMC]
Rodríguez-Giraldo M, González-Reyes RE, Ramírez-Guerrero S, Bonilla-Trilleras CE, Guardo-Maya S, Nava-Mesa MO (2022) Astrocytes as a therapeutic target in Alzheimer’s disease-comprehensive review and recent developments. International Journal of Molecular Sciences 23(21): 13630. https://doi.org/10.3390/ijms232113630 [PubMed] [PMC]
Roy A, Sharma S, Nag TC, Katyal J, Gupta YK, Jain S (2022) Cognitive dysfunction and anxiety resulting from synaptic downscaling, hippocampal atrophy, and ventricular enlargement with intracerebroventricular streptozotocin injection in male Wistar rats. Neurotoxicity Research 40(6): 2179–2202. https://doi.org/10.1007/s12640-022-00563-x [PubMed]
Salman MM, Kitchen P, Halsey A, Wang MX, Törnroth-Horsefield S, Conner AC, Badaut J, Iliff JJ, Bill RM (2022) Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain 145(1): 64–75. https://doi.org/10.1093/brain/awab311 [PubMed] [PMC]
Sasaki K, Fujita H, Sato T, Kato S, Takahashi Y, Takeshita Y, Kanda T, Saito T, Saido TC, Hattori S, Hozumi Y, Yamada Y, Waki H (2024) GLP-1 receptor signaling restores aquaporin 4 subcellular polarization in reactive astrocytes and promotes amyloid β clearance in a mouse model of Alzheimer’s disease. Biochemical and Biophysical Research Communications 741: 151016. https://doi.org/10.1016/j.bbrc.2024.151016 [PubMed]
Sauvé F, Ternier G, Dewisme J, Lebouvier T, Dupré E, Danis C, Rasika S, Kim Y-B, Ciofi P, Giacobini P, Buée L, Landrieu I, Pasquier F, Maurage C-A, Nogueiras R, Schwaninger M, Prevot V (2022) Tanycytes are degraded in Alzheimer’s disease, disrupting the brain-to-blood efflux of Tau. Preprint from medRxiv: PPR: PPR496366. https://doi.org/10.1101/2022.05.04.22274181
Silva I, Silva J, Ferreira R, Trigo D (2021) Glymphatic system, AQP4, and their implications in Alzheimer’s disease. Neurological Research and Practice 3(1): 5. https://doi.org/10.1186/s42466-021-00102-7 [PubMed] [PMC]
Smith AJ, Duan T, Verkman AS (2019) Aquaporin-4 reduces neuropathology in a mouse model of Alzheimer’s disease by remodeling peri-plaque astrocyte structure. Acta Neuropathologica Communications 7(1): 74. https://doi.org/10.1186/s40478-019-0728-0 [PubMed] [PMC]
Stavrovskaya AV, Voronkov DN, Shestakova EA, Gushchina AS, Olshansky AS, Yamshikova NG (2019) Streptozocin-induced Alzheimer’s disease as an independent risk factor for the development of hyperglycemia in Wistar rats. Problems of Endocrinology 65(5): 351–361. https://doi.org/10.14341/probl12126 [PubMed]
Teixeira LCR, Luizon MR, Gomes KB (2025) Exploring the role of GLP-1 receptor agonists in Alzheimer’s disease: A review of preclinical and clinical evidence. Receptors 4: 2. https://doi.org/10.3390/receptors4010002
Todd KL, Brighton T, Norton ES, Schick S, Elkins W, Pletnikova O, Fortinsky RH, Troncoso JC, Molfese PJ, Resnick SM, Conover JC, for the Alzheimer’s Disease Neuroimaging Initiative (2018) Ventricular and periventricular anomalies in the aging and cognitively impaired brain. Frontiers in Aging Neuroscience 9: 445. https://doi.org/10.3389/fnagi.2017.00445 [PubMed] [PMC]
Urkon M, Ferencz E, Szász JA, Szabo MIM, Orbán-Kis K, Szatmári S, Nagy EE (2025) Antidiabetic GLP-1 receptor agonists have neuroprotective properties in experimental animal models of Alzheimer’s disease. Pharmaceuticals 18(5): 614. https://doi.org/10.3390/ph18050614 [PubMed] [PMC]
Valenza M, Facchinetti R, Steardo L, Scuderi C (2020) Altered waste disposal system in aging and Alzheimer’s disease: Focus on astrocytic aquaporin-4. Frontiers in Pharmacology 10: 1656. https://doi.org/10.3389/fphar.2019.01656 [PubMed] [PMC]
Verkhratsky A, Butt A, Li B, Illes P, Zorec R, Semyanov A, Tang Y, Sofroniew MV (2023) Astrocytes in human central nervous system diseases: a frontier for new therapies. Signal Transduction and Targeted Therapy 8(2): 396. https://doi.org/10.1038/s41392-023-01628-9[PubMed] [PMC]
Voronkov D, Stavrovskaya A, Gushchina A, Olshansky A (2021) Alterations in tanycytes and related cell populations of arcuate nucleus in streptozotocin-induced Alzheimer disease model. Bulletin of Russian State Medical University 5: 11-19. https://doi.org/10.24075/brsmu.2021.050 [in Russian]
Voronkov DN, Stavrovskaya AV, Potapov IA, Guschina AS, Olshanskiy AS (2023) Glial reaction in a neuroinflammatory model of Parkinson’s disease. Bulletin of Experimental Biology and Medicine 174(5): 693–698. https://doi.org/10.1007/s10517-023-05772-8 [PubMed]
Voronkov DN, Stavrovskaya AV, Stelmashook EV, Genrikhs EE, Isaev NK (2019) Neurodegenerative changes in rat brain in streptozotocin model of Alzheimer’s disease. Bulletin of Experimental Biology and Medicine 166: 793–796. https://doi.org/10.1007/s10517-019-04442-y [in Russian]
Yoon JH, Hwang J, Son SU, Choi J, You S-W, Park H, Cha S-Y, Maeng S (2023) How can insulin resistance cause Alzheimer’s disease? International Journal of Molecular Sciences 24(4): 3506. https://doi.org/10.3390/ijms24043506 [PubMed] [PMC]
Zeppenfeld DM, Simon M, Haswell JD, D’Abreo D, Murchison C, Quinn JF, Grafe MR, Woltjer RL, Kaye J, Iliff JJ (2017) Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in Aging Brains. JAMA Neurology 74(1): 91-99. https://doi.org/10.1001/jamaneurol.2016.4370 [PubMed]
Zhang Q, Liu C, Shi R, Zhou S, Shan H, Deng L, Chen T, Guo Y, Zhang Z, Yang G-Y, Wang Y, Tang Y (2022) Blocking C3d+/GFAP+ A1 astrocyte conversion with semaglutide attenuates blood-brain barrier disruption in mice after ischemic stroke. Aging and Disease 13: 943. https://doi.org/10.14336/ad.2021.1029 [PubMed] [PMC]
Zhang T, Ai D, Wei P, Xu Y, Bi Z, Ma F, Li F, Chen X, Zhang Z, Zou X, Guo Z, Zhao Y, Li J-L, Ye M, Feng Z, Zhang X, Zheng L, Yu J, Li C, Tu T, Zeng H, Lei J, Zhang H, Hong T, Zhang L, Luo B, Li Z, Xing C, Jia C, Li L, Sun W, Ge W (2024) The subcommissural organ regulates brain development via secreted peptides. Nature Neuroscience 27(6): 1103–1115. https://doi.org/10.1038/s41593-024-01639-x [PubMed]
Zhang X, Wang Y, Jiao B, Wang Z, Shi J, Zhang Y, Bai X, Li Z, Li S, Bai R, Sui B (2023) Glymphatic system impairment in Alzheimer’s disease: associations with perivascular space volume and cognitive function. European Radiology 34(2): 1314–1323. https://doi.org/10.1007/s00330-023-10122-3 [PubMed]
Zhou M, Zhong S, Verkhratsky A (2024) Astrocyte syncytium: from neonatal genesis to aging degeneration. Neural Regeneration Research 19(2): 395–396. https://doi.org/10.4103/1673-5374.379047 [PubMed] [PMC]
Загрузки
Опубликован
Как цитировать
Выпуск
Раздел
Лицензия
Copyright (c) 2025 Voronkov DN, Stavrovskaya AV, Pavlova AK, Potapov IA, Olshansky AS, Sukhorukov VS

Это произведение доступно по лицензии Creative Commons «Attribution» («Атрибуция») 4.0 Всемирная.
 Русский
Русский
 English
English

