Эффективность и безопасность моноклональных антител, применяемых в терапии болезни Альцгеймера
DOI:
https://doi.org/10.18413/rrpharmacology.11.842Аннотация
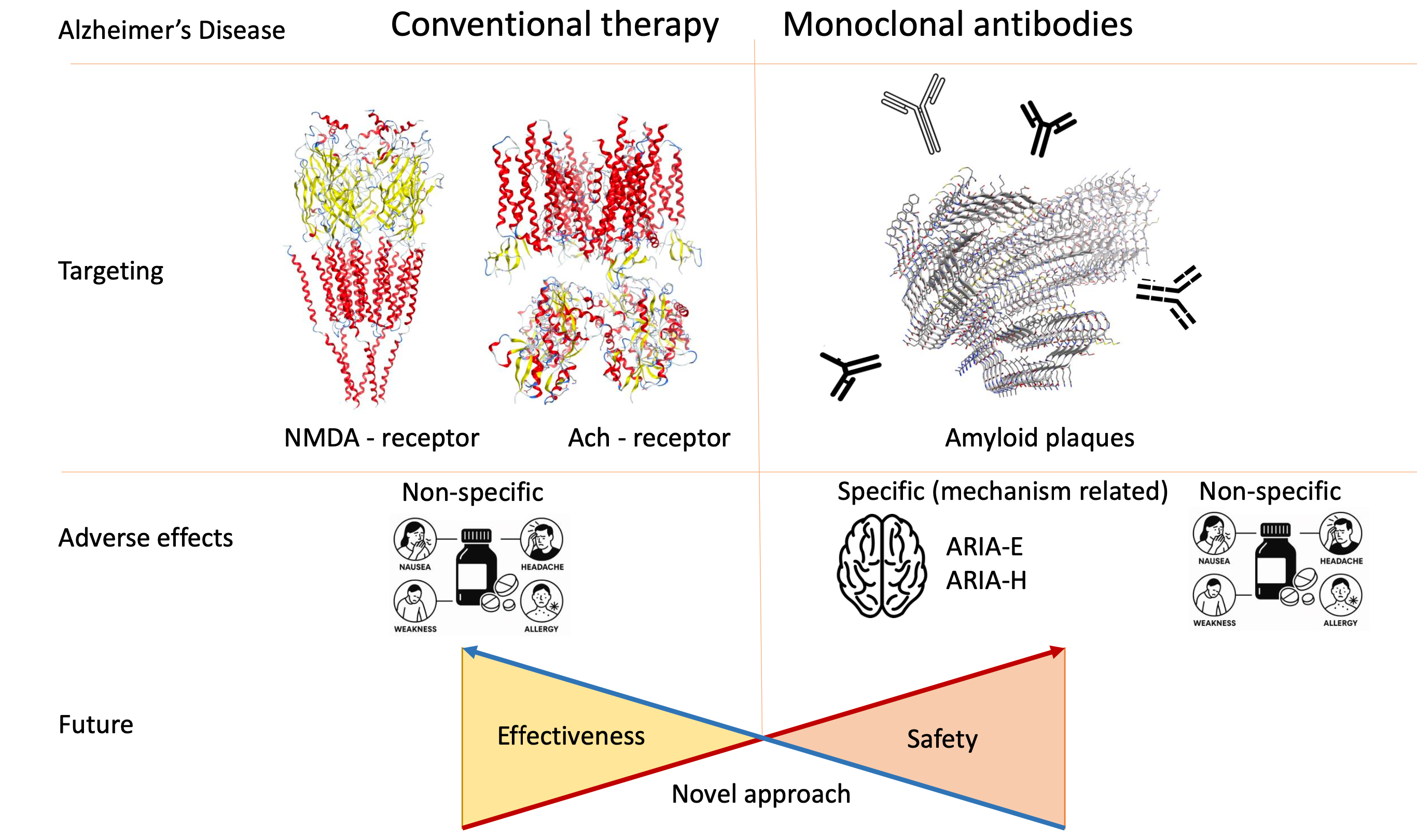
Введение: Достижения в области молекулярной биологии и биотехнологии способствовали разработке новых терапевтических стратегий лечения болезни Альцгеймера (БА). Значительный интерес среди них представляют моноклональные антитела. Данный класс препаратов привлекает внимание благодаря своей способности целенаправленно влиять на ключевые патологические процессы, а именно накопление амилоидных бляшек и тау-белка в головном мозге, что открывает перспективы для патогенетического подхода к терапии. Целью данного исследования является систематизация данных об эффективности, нежелательных явлениях и показателях смертности при применении моноклональных антител на основе результатов клинических испытаний в сопоставлении с традиционной терапией.
Материалы и методы: Для проведения анализа были найдены все зарегистрированные клинические исследования, посвященные применению моноклональных антител для терапии БА, в базе данных ClinicalTrials.gov. Данные о нежелательных явлениях были получены из публикаций, относящихся к клиническим исследованиям III фазы; в итоговый анализ вошла 21 статья. Сведения о традиционной терапии были получены из актуальных клинических рекомендаций по лечению БА.
Результаты: Проведенный анализ выявил, что частота серьезных нежелательных явлений и уровень смертности существенно варьируются в зависимости от конкретного моноклонального антитела. В большинстве случаев регистрируемые побочные эффекты не являются специфичными для применяемых препаратов. В связи с этим установить прямую причинно-следственную связь между приемом лекарственного средства и развитием определенных нежелательных явлений, а также провести адекватное сравнение профилей безопасности различных препаратов затруднительно ввиду различий в их механизмах действия и рекомендуемых дозировках. Магнитно-резонансная томография с оценкой амилоид-связанных аномалий визуализации (ARIA) по типу ARIA-E и ARIA-H представляется наиболее перспективным инструментом для сравнительной оценки антител, однако данный метод пока не обладает достаточной корреляцией с клинической симптоматикой. Кроме того, рекомендуется отдельно учитывать реакции, связанные с инфузией препаратов.
Заключение: Применение патогенетической терапии моноклональными антителами позволит в будущем повысить качество лечения БА. Однако на сегодняшний день не существует унифицированных протоколов для оценки эффективности препаратов и их сравнения. Оценка побочных эффектов и определение препарат-специфичных нежелательных реакций необходимы для повышения безопасности лечения пациентов с БА.
Графическая аннотация
Ключевые слова:
болезнь Альцгеймера, терапия болезни Альцгеймера, эффективность, безопасность, моноклональные антитела, ARIA-H, ARIA-EБиблиографические ссылки
Alawode DOT, Heslegrave AJ, Fox NC, Zetterberg H (2021) Donanemab removes Alzheimer’s plaques: what is special about its target? The Lancet Healthy Longevity 2(7): e395–e396. https://doi.org/10.1016/S2666-7568(21)00144-6 [PubMed]
NICE guideline (2018) Dementia: assessment, management and support for people living with dementia and their carers. https://www.nice.org.uk/guidance/ng97 [accessed on 30 July 2025]
NHS (2025) Alzheimer’s disease - Treatment. https://www.nhs.uk/conditions/alzheimers-disease/treatment/ [accessed on 30 July 2025]
FDA (2024) Early Alzheimer’s Disease: Developing Drugs for Treatment. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/early-alzheimers-disease-developing-drugs-treatment [accessed on 30 July 2025]
National Institute on Aging (2025) How Is Alzheimer’s Disease Treated? https://www.nia.nih.gov/health/alzheimers-treatment/how-alzheimers-disease-treated [accessed on 30 July 2025]
Alzheimer’s Society (2025) Medication for dementia symptoms. https://www.alzheimers.org.uk/about-dementia/treatments/dementia-medication/medication-dementia-symptoms [accessed on 30 July 2025]
Alzheimer’s Association (2025) Medications for Memory, Cognition & Dementia-Related Behaviors. https://www.alz.org/alzheimers-dementia/treatments/medications-for-memory [accessed on 30 July 2025]
Bateman RJ, Smith J, Donohue MC, Delmar P, Abbas R, Salloway S, Wojtowicz J, Blennow K, Bittner T, Black SE, Klein G, Boada M, Grimmer T, Tamaoka A, Perry RJ, Turner RS, Watson D, Woodward M, Thanasopoulou A, Lane C, Baudler M, Fox NC, Cummings JL, Fontoura P, Doody RS (2023) Two phase 3 trials of gantenerumab in early alzheimer’s disease. New England Journal of Medicine 389(20): 1862–1876. https://doi.org/10.1056/NEJMOA2304430 [PubMed] [PMC]
Battle CE, Abdul-Rahim AH, Shenkin SD, Hewitt J, Quinn TJ (2021) Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: a network meta-analysis. Cochrane Database of Systematic Reviews 2(2): CD013306. https://doi.org/10.1002/14651858.CD013306.PUB2 [PubMed] [PMC]
Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, Wilkinson D, Holmes C, Nicoll JAR (2008) Consequence of Aβ immunization on the vasculature of human Alzheimer’s disease brain. Brain 131(Pt 12): 3299–3310. https://doi.org/10.1093/BRAIN/AWN261 [PubMed]
Bohrmann B, Baumann K, Benz J, Gerber F, Huber W, Knoflach F, Messer J, Oroszlan K, Rauchenberger R, Richter WF, Rothe C, Urban M, Bardroff M, Winter M, Nordstedt C, Loetscher H (2012) Gantenerumab: A novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β. Journal of Alzheimer’s Disease 28(1): 49–69. https://doi.org/10.3233/JAD-2011-110977 [PubMed]
Budd Haeberlein S, Aisen PS, Barkhof F, Chalkias S, Chen T, Cohen S, Dent G, Hansson O, Harrison K, von Hehn C, Iwatsubo T, Mallinckrodt C, Mummery CJ, Muralidharan KK, Nestorov I, Nisenbaum L, Rajagovindan R, Skordos L, Tian Y, van Dyck CH, Vellas B, Wu S, Zhu Y, Sandrock A (2022) Two randomized phase 3 studies of aducanumab in early alzheimer’s disease. Journal of Prevention of Alzheimer’s Disease 9(2): 197–210. https://doi.org/10.14283/jpad.2022.30 [PubMed]
Cáceres MC, Guerrero-Martín J, Pérez-Civantos D, Palomo-López P, Delgado-Mingorance JI, Durán-Gómez N (2019) The importance of early identification of infusion-related reactions to monoclonal antibodies. Therapeutics and Clinical Risk Management 15: 965–977. https://doi.org/10.2147/TCRM.S204909 [PubMed] [PMC]
Cummings JL, Cohen S, van Dyck CH, Brody M, Curtis C, Cho W, Ward M, Friesenhahn M, Rabe C, Brunstein F, Quartino A, Honigberg LA, Fuji RN, Clayton D, Mortensen D, Ho C, Paul R (2018) ABBY: A phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease. Neurology 90(21): e1889-e1897. https://doi.org/10.1212/WNL.0000000000005550 [PubMed] [PMC]
Cummings J, Rabinovici GD, Atri A, Aisen P, Apostolova LG, Hendrix S, Sabbagh M, Selkoe D, Weiner M, Salloway S (2022) Aducanumab: Appropriate use recommendations update. Journal of Prevention of Alzheimer’s Disease 9(2): 221–230. https://doi.org/10.14283/jpad.2022.34 [PubMed] [PMC]
Cummings J, Apostolova L, Rabinovici GD, Atri A, Aisen P, Greenberg S, Hendrix S, Selkoe D, Weiner M, Petersen RC, Salloway S (2023) Lecanemab: Appropriate use recommendations. Journal of Prevention of Alzheimer’s Disease 10(3): 362–377. https://doi.org/10.14283/jpad.2023.30 [PubMed] [PMC]
Cummings J, Osse AML, Cammann D, Powell J, Chen J (2024) Anti-amyloid monoclonal antibodies for the treatment of alzheimer’s disease. BioDrugs 38(1): 5–22. https://doi.org/10.1007/S40259-023-00633-2 [PubMed] [PMC]
Cummings JL (2025) Maximizing the benefit and managing the risk of anti-amyloid monoclonal antibody therapy for Alzheimer’s disease: Strategies and research directions. Neurotherapeutics 22(3): e00570. https://doi.org/10.1016/j.neurot.2025.e00570 [PubMed] [PMC]
Day GS, Scarmeas N, Dubinsky R, Coerver K, Mostacero A, West B, Wessels SR, Armstrong MJ (2022) Aducanumab use in symptomatic alzheimer disease evidence in focus [retired]. Neurology 98(15): 619–631. https://doi.org/10.1212/WNL.0000000000200176 [PubMed] [PMC]
Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS, Siemers E, Liu-Seifert H, Mohs R (2014) Phase 3 trials of solanezumab for mild-to-moderate alzheimer’s disease. New England Journal of Medicine 370(4): 311–321. https://doi.org/10.1056/NEJMOA1312889 [PubMed]
Finke JM, Banks WA (2017) Modulators of IgG penetration through the blood-brain barrier: Implications for Alzheimer’s disease immunotherapy. Human Antibodies 25(3-4): 131–146. https://doi.org/10.3233/HAB-160306 [PubMed]
Foley KE, Wilcock DM (2024) Three major effects of APOEε4 on Aβ immunotherapy induced ARIA. Frontiers in Aging Neuroscience 16: 1412006. https://doi.org/10.3389/FNAGI.2024.1412006 [PubMed] [PMC]
Forner S, Kawauchi S, Balderrama-Gutierrez G, Kramár EA, Matheos DP, Phan J, Javonillo DI, Tran KM, Hingco E, da Cunha C, Rezaie N, Alcantara JA, Baglietto-Vargas D, Jansen C, Neumann J, Wood MA, MacGregor GR, Mortazavi A, Tenner AJ, LaFerla FM, Green KN (2021) Systematic phenotyping and characterization of the 5xFAD mouse model of Alzheimer’s disease. Scientific Data 8(1): 270. https://doi.org/10.1038/S41597-021-01054-Y [PubMed] [PMC]
Gnoth K, Piechotta A, Kleinschmidt M, Konrath S, Schenk M, Taudte N, Ramsbeck D, Rieckmann V, Geissler S, Eichentopf R, Barendrecht S, Hartlage-Rübsamen M, Demuth HU, Roßner S, Cynis H, Rahfeld JU, Schilling S (2020) Targeting isoaspartate-modified Aβ rescues behavioral deficits in transgenic mice with Alzheimer’s disease-like pathology. Alzheimer’s Research and Therapy 12(1): 149. https://doi.org/10.1186/S13195-020-00719-X [PubMed] [PMC]
Guthrie H, Honig LS, Lin H, Sink KM, Blondeau K, Quartino A, Dolton M, Carrasco-Triguero M, Lian Q, Bittner T, Clayton D, Smith J, Ostrowitzki S (2020) Safety, tolerability, and pharmacokinetics of crenezumab in patients with mild-to-moderate alzheimer’s disease treated with escalating doses for up to 133 weeks. Journal of Alzheimer’s Disease 76(3): 967–979. https://doi.org/10.3233/JAD-200134[PubMed] [PMC]
Haddad HW, Malone GW, Comardelle NJ, Degueure AE, Kaye AM, Kaye AD (2022) Aducanumab, a novel anti-amyloid monoclonal antibody, for the treatment of alzheimer’s disease: a comprehensive review. Health Psychology Research 10(1): 31925. https://doi.org/10.52965/001C.31925 [PubMed] [PMC]
Hampel H, Elhage A, Cho M, Apostolova LG, Nicoll JAR, Atri A (2023) Amyloid-related imaging abnormalities (ARIA): radiological, biological and clinical characteristics. Brain 146(11): 4414–4424. https://doi.org/10.1093/BRAIN/AWAD188 [PubMed] [PMC]
Honig LS, Vellas B, Woodward M, Boada M, Bullock R, Borrie M, Hager K, Andreasen N, Scarpini E, Liu-Seifert H, Case M, Dean RA, Hake A, Sundell K, Poole Hoffmann V, Carlson C, Khanna R, Mintun M, DeMattos R, Selzler KJ, Siemers E (2018) Trial of solanezumab for mild dementia due to alzheimer’s disease. New England Journal of Medicine 378(4): 321–330. https://doi.org/10.1056/NEJMOA1705971 [PubMed]
Jack CR, Wiste HJ, Algeciras-Schimnich A, Figdore DJ, Schwarz CG, Lowe VJ, Ramanan VK, Vemuri P, Mielke MM, Knopman DS, Graff-Radford J, Boeve BF, Kantarci K, Cogswell PM, Senjem ML, Gunter JL, Therneau TM, Petersen RC (2023) Predicting amyloid PET and tau PET stages with plasma biomarkers. Brain 146(5): 2029–2044. https://doi.org/10.1093/BRAIN/AWAD042 [PubMed] [PMC]
Jack CR, Andrews JS, Beach TG, Buracchio T, Dunn B, Graf A, Hansson O, Ho C, Jagust W, McDade E, Molinuevo JL, Okonkwo OC, Pani L, Rafii MS, Scheltens P, Siemers E, Snyder HM, Sperling R, Teunissen CE, Carrillo MC (2024) Revised criteria for diagnosis and staging of Alzheimer’s disease: Alzheimer’s Association Workgroup. Alzheimer’s and Dementia 20(8): 5143–5169. https://doi.org/10.1002/ALZ.13859 [PubMed] [PMC]
Janowicz PW, Leinenga G, Götz J, Nisbet RM (2019) Ultrasound-mediated blood-brain barrier opening enhances delivery of therapeutically relevant formats of a tau-specific antibody. Scientific Reports 9(1): 9255. https://doi.org/10.1038/S41598-019-45577-2 [PubMed] [PMC]
Klein G, Delmar P, Voyle N, Rehal S, Hofmann C, Abi-Saab D, Andjelkovic M, Ristic S, Wang G, Bateman R, Kerchner GA, Baudler M, Fontoura P, Doody R (2019) Gantenerumab reduces amyloid-β plaques in patients with prodromal to moderate Alzheimer’s disease: A PET substudy interim analysis. Alzheimer’s Research and Therapy 11(1): 101. https://doi.org/10.1186/S13195-019-0559-Z [PubMed] [PMC]
Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, Small GW, Miller B, Stevens JC (2001) Practice parameter: Diagnosis of dementia (an evidence-based review): Report of the quality standards subcommittee of the american academy of neurology. Neurology 56(9): 1143–1153. https://doi.org/10.1212/WNL.56.9.1143 [PubMed]
Kozin SA, Cheglakov IB, Ovsepyan AA, Telegin GB, Tsvetkov PO, Lisitsa AV, Makarov AA (2013) Peripherally applied synthetic peptide isoAsp7-Aβ(1-42) triggers cerebral β-amyloidosis. Neurotoxicity Research 24(3): 370–376. https://doi.org/10.1007/S12640-013-9399-Y[PubMed]
Kozin SA, Barykin EP, Telegin GB, Chernov AS, Adzhubei AA, Radko SP, Mitkevich VA, Makarov AA (2018) Intravenously injected amyloid-β peptide with isomerized Asp7 and phosphorylated Ser8 residues inhibits cerebral β-amyloidosis in AβPP/PS1 transgenic mice model of Alzheimer’s disease. Frontiers in Neuroscience 12: 518. https://doi.org/10.3389/FNINS.2018.00518 [PubMed] [PMC]
Malik B, Ghatol A (2023) Understanding how monoclonal antibodies work. StatPearls. [PubMed]
Masters CL, Selkoe DJ (2012) Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease. Cold Spring Harbor Perspectives in Medicine 2(6): a006262. https://doi.org/10.1101/CSHPERSPECT.A006262 [PubMed] [PMC]
Menegaz de Almeida A, Leite M, Lopes LM, Gomes Lima P, Siegloch Barros ML, Rocha Pinheiro S, Andrade Í, Viana P, Morbach V, Marinheiro G, de Oliveira R, Pinheiro AC (2024) Gantenerumab for early Alzheimer’s disease: a systematic review and meta-analysis. Expert Review of Neurotherapeutics 24(9): 929–936. https://doi.org/10.1080/14737175.2024.2367016 [PubMed]
Mitkevich VA, Barykin EP, Eremina S, Pani B, Katkova-Zhukotskaya O, Polshakov VI, Adzhubei AA, Kozin SA, Mironov AS, Makarov AA, Nudler E (2023) Zn-dependent β-amyloid aggregation and its reversal by the tetrapeptide HAEE. Aging and Disease 14(2): 309–314. https://doi.org/10.14336/AD.2022.0827 [PubMed] [PMC]
Mitkevich VA, Petrushanko IY, Yegorov YE, Simonenko O V., Vishnyakova KS, Kulikova AA, Tsvetkov PO, Makarov AA, Kozin SA (2013) Isomerization of Asp7 leads to increased toxic effect of amyloid-b42 on human neuronal cells. Cell Death and Disease 4(11): e939. https://doi.org/10.1038/CDDIS.2013.492 [PubMed] [PMC]
Ostrowitzki S, Lasser RA, Dorflinger E, Scheltens P, Barkhof F, Nikolcheva T, Ashford E, Retout S, Hofmann C, Delmar P, Klein G, Andjelkovic M, Dubois B, Boada M, Blennow K, Santarelli L, Fontoura P (2017) A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimer’s Research and Therapy 9(1): 95. https://doi.org/10.1186/S13195-017-0318-Y [PubMed] [PMC]
Ostrowitzki S, Bittner T, Sink KM, Mackey H, Rabe C, Honig LS, Cassetta E, Woodward M, Boada M, Van Dyck CH, Grimmer T, Selkoe DJ, Schneider A, Blondeau K, Hu N, Quartino A, Clayton D, Dolton M, Dang Y, Ostaszewski B, Sanabria-Bohórquez SM, Rabbia M, Toth B, Eichenlaub U, Smith J, Honigberg LA, Doody RS (2022) Evaluating the safety and efficacy of crenezumab vs placebo in adults with early alzheimer disease: two phase 3 randomized placebo-controlled trials. JAMA Neurology 79(11): 1113–1121. https://doi.org/10.1001/JAMANEUROL.2022.2909 [PubMed] [PMC]
Perneczky R, Dom G, Chan A, Falkai P, Bassetti C (2024) Anti-amyloid antibody treatments for Alzheimer’s disease. European Journal of Neurology 31(2): e16049. https://doi.org/10.1111/ENE.16049 [PubMed] [PMC]
Perneczky R, Jessen F, Grimmer T, Levin J, Flöel A, Peters O, Froelich L (2023) Anti-amyloid antibody therapies in Alzheimer’s disease. Brain 146(3): 842–849. https://doi.org/10.1093/BRAIN/AWAD005 [PubMed]
Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, Gronseth GS, Marson D, Pringsheim T, Day GS, Sager M, Stevens J, Rae-Grant A (2018) Practice guideline update summary: Mild cognitive impairment report of theguideline development, dissemination, and implementation. Neurology 90(3): 126–135. https://doi.org/10.1212/WNL.0000000000004826 [PubMed] [PMC]
Plotkin SS, Cashman NR (2020) Passive immunotherapies targeting Aβ and tau in Alzheimer’s disease. Neurobiology of Disease 144: 105010. https://doi.org/10.1016/j.nbd.2020.105010 [PubMed] [PMC]
Qiao Y, Gu J, Yu M, Chi Y, Ma Y (2024) Comparative efficacy and safety of monoclonal antibodies for cognitive decline in patients with alzheimer’s disease: a systematic review and network meta-analysis. CNS Drugs 38(3): 169–192. https://doi.org/10.1007/S40263-024-01067-2 [PubMed]
Rahman A, Hossen MA, Chowdhury MFI, Bari S, Tamanna N, Sultana SS, Haque SN, Al Masud A, Saif-Ur-Rahman KM (2023) Aducanumab for the treatment of Alzheimer’s disease: a systematic review. Psychogeriatrics 23(3): 512–522. https://doi.org/10.1111/PSYG.12944 [PubMed] [PMC]
Rashad A, Rasool A, Shaheryar M, Sarfraz A, Sarfraz Z, Robles-Velasco K, Cherrez-Ojeda I (2023) Donanemab for alzheimer’s disease: A systematic review of clinical trials. Healthcare (Switzerland) 11(1): 32. https://doi.org/10.3390/HEALTHCARE11010032 [PubMed] [PMC]
Reisberg B, Ferris SH, De Leon MJ, Crook T (1982) The global deterioration scale for assessment of primary degenerative dementia. American Journal of Psychiatry 139(9): 1136–1139. https://doi.org/10.1176/AJP.139.9.1136 [PubMed]
Robert P, Ferris S, Gauthier S, Ihl R, Winblad B, Tennigkeit F (2010) Review of Alzheimer’s disease scales: Is there a need for a new multi-domain scale for therapy evaluation in medical practice? Alzheimer’s Research and Therapy 2(4): 24. https://doi.org/10.1186/ALZRT48[PubMed] [PMC]
Rofo F, Meier SR, Metzendorf NG, Morrison JI, Petrovic A, Syvänen S, Sehlin D, Hultqvist G (2022) A brain-targeting bispecific-multivalent antibody clears soluble amyloid-beta aggregates in alzheimer’s disease mice. Neurotherapeutics 19(5): 1588–1602. https://doi.org/10.1007/s13311-022-01283-y [PubMed] [PMC]
Rosen WG, Mohs RC, Davis KL (1984) A new rating scale for Alzheimer’s disease. American Journal of Psychiatry 141(11): 1356-1364. https://doi.org/10.1176/AJP.141.11.1356 [PubMed]
Rozankovic PB, Rozankovic M, Badzak J, Stojic M, Sporis IS (2021) Impact of donepezil and memantine on behavioral and psychological symptoms of alzheimer disease: Six-month open-label study. Cognitive and Behavioral Neurology 34(4): 288–294. https://doi.org/10.1097/WNN.0000000000000285 [PubMed]
Salloway S, Pain A, Lee E, Papka M, Ferguson MB, Wang H, Hu H, Lu M, Oru E, Ardayfio PA, Hoban DB, Collins EC, Brooks DA, Sims JR (2025) TRAILBLAZER-ALZ 4: A phase 3 trial comparing donanemab with aducanumab on amyloid plaque clearance in early, symptomatic Alzheimer’s disease. Alzheimer’s and Dementia 21(5): e70293. https://doi.org/10.1002/ALZ.70293 [PubMed] [PMC]
Salloway S, Honigberg LA, Cho W, Ward M, Friesenhahn M, Brunstein F, Quartino A, Clayton D, Mortensen D, Bittner T, Ho C, Rabe C, Schauer SP, Wildsmith KR, Fuji RN, Suliman S, Reiman EM, Chen K, Paul R (2018) Amyloid positron emission tomography and cerebrospinal fluid results from a crenezumab anti-amyloid-beta antibody double-blind, placebo-controlled, randomized phase II study in mild-to-moderate Alzheimer’s disease (BLAZE). Alzheimer’s Research and Therapy 10(1): 96. https://doi.org/10.1186/S13195-018-0424-5/FIGURES/5 [PubMed] [PMC]
Salloway S, Farlow M, McDade E, Clifford DB, Wang G, Llibre-Guerra JJ, Hitchcock JM, Mills SL, Santacruz AM, Aschenbrenner AJ, Hassenstab J, Benzinger TLS, Gordon BA, Fagan AM, Coalier KA, Cruchaga C, Goate AA, Perrin RJ, Xiong C, Li Y, Morris JC, Snider BJ, Mummery C, Surti GM, Hannequin D, Wallon D, Berman SB, Lah JJ, Jimenez-Velazquez IZ, Roberson ED, van Dyck CH, Honig LS, Sánchez-Valle R, Brooks WS, Gauthier S, Galasko DR, Masters CL, Brosch JR, Hsiung GYR, Jayadev S, Formaglio M, Masellis M, Clarnette R, Pariente J, Dubois B, Pasquier F, Jack CR, Koeppe R, Snyder PJ, Aisen PS, Thomas RG, Berry SM, Wendelberger BA, Andersen SW, Holdridge KC, Mintun MA, Yaari R, Sims JR, Baudler M, Delmar P, Doody RS, Fontoura P, Giacobino C, Kerchner GA, Bateman RJ, Formaglio M, Mills SL, Pariente J, van Dyck CH (2021) A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer’s disease. Nature Medicine 27(7): 1187–1196. https://doi.org/10.1038/S41591-021-01369-8 [PubMed] [PMC]
Sevigny J, Chiao P, Bussière T, Weinreb PH, Williams L, Maier M, Dunstan R, Salloway S, Chen T, Ling Y, O’Gorman J, Qian F, Arastu M, Li M, Chollate S, Brennan MS, Quintero-Monzon O, Scannevin RH, Arnold HM, Engber T, Rhodes K, Ferrero J, Hang Y, Mikulskis A, Grimm J, Hock C, Nitsch RM, Sandrock A (2016) The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537(7618): 50–56. https://doi.org/10.1038/NATURE19323 [PubMed]
Shaji KS, Sivakumar PT, Rao GP, Paul N (2018) Clinical practice guidelines for management of dementia. Indian Journal of Psychiatry 60(Suppl 3): S312–S328. https://doi.org/10.4103/0019-5545.224472 [PubMed] [PMC]
Shimizu T, Watanabe A, Ogawara M, Mori H, Shirasawa T (2000) Isoaspartate formation and neurodegeneration in Alzheimer’s disease. Archives of Biochemistry and Biophysics 381(2): 225–234. https://doi.org/10.1006/abbi.2000.1955 [PubMed]
Sims JR, Zimmer JA, Evans CD, Lu M, Ardayfio P, Sparks JD, Wessels AM, Shcherbinin S, Wang H, Monkul Nery ES, Collins EC, Solomon P, Salloway S, Apostolova LG, Hansson O, Ritchie C, Brooks DA, Mintun M, Skovronsky DM (2023) Donanemab in early symptomatic alzheimer disease: The TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330(6): 512–527. https://doi.org/10.1001/JAMA.2023.13239 [PubMed] [PMC]
Söderberg L, Johannesson M, Nygren P, Laudon H, Eriksson F, Osswald G, Möller C, Lannfelt L (2023) Lecanemab, aducanumab, and gantenerumab – binding profiles to different forms of amyloid-beta might explain efficacy and side effects in clinical trials for alzheimer’s disease. Neurotherapeutics 20(1): 195–206. https://doi.org/10.1007/s13311-022-01308-6 [PubMed] [PMC]
Sperling RA, Donohue MC, Raman R, Rafii MS, Johnson K, Masters CL, van Dyck CH, Iwatsubo T, Marshall GA, Yaari R, Mancini M, Holdridge KC, Case M, Sims JR, Aisen PS (2023) Trial of solanezumab in preclinical alzheimer’s disease. New England Journal of Medicine 389(12): 1096–1107. https://doi.org/10.1056/NEJMOA2305032/SUPPL_FILE/NEJMOA2305032_DATA-SHARING.PDF [PubMed] [PMC]
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH (2011) Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the national institute on aging-alzheimer’s association workgroups on diagnostic guidelines for alzheimer’s disease. Alzheimer’s and Dementia 7(3): 280–292. https://doi.org/10.1016/J.JALZ.2011.03.003[PubMed] [PMC]
Varadharajan A, Davis AD, Ghosh A, Jagtap T, Xavier A, Menon AJ, Roy D, Gandhi S, Gregor T (2023) Guidelines for pharmacotherapy in Alzheimer’s disease – A primer on FDA-approved drugs. Journal of Neurosciences in Rural Practice 14(4): 566–573. https://doi.org/10.25259/JNRP_356_2023 [PubMed] [PMC]
Zatsepina OG, Kechko OI, Mitkevich VA, Kozin SA, Yurinskaya MM, Vinokurov MG, Serebryakova M V., Rezvykh AP, Evgen’Ev MB, Makarov AA (2018) Amyloid-β with isomerized Asp7 cytotoxicity is coupled to protein phosphorylation. Scientific Reports 8(1): 3518. https://doi.org/10.1038/S41598-018-21815-X [PubMed] [PMC]
Загрузки
Опубликован
Как цитировать
Выпуск
Раздел
Лицензия
Copyright (c) 2025 Koledova YuV, Bychkovskii NI, Mitkevich VA, Makarov AA, Poluektov YuM

Это произведение доступно по лицензии Creative Commons «Attribution» («Атрибуция») 4.0 Всемирная.
 Русский
Русский
 English
English

