Evaluation of safety, efficacy and pharmacokinetics of Eltrombopag in patients with chronic immune thrombocytopenia: Meta-analysis of randomized controlled trials
DOI:
https://doi.org/10.18413/rrpharmacology.9.10033Abstract
Introduction: Immune thrombocytopenia (ITP) is a complex autoimmune syndrome associated with low platelet count. Eltrombopag is an oral thrombopoietin receptor agonist that used in the treatment of chronic ITP.
The aim of the study: The present meta-analysis is to evaluate the safety and efficiency of Eltrombopag in theprevention and therapy of ITP.
Materials and Methods: The analysis was performed according to the PRISMA guideline with use of Excerpta MedicaDatabase (EMBASE) as well as Web of Science and the Cochrane (CENTAL) databases.
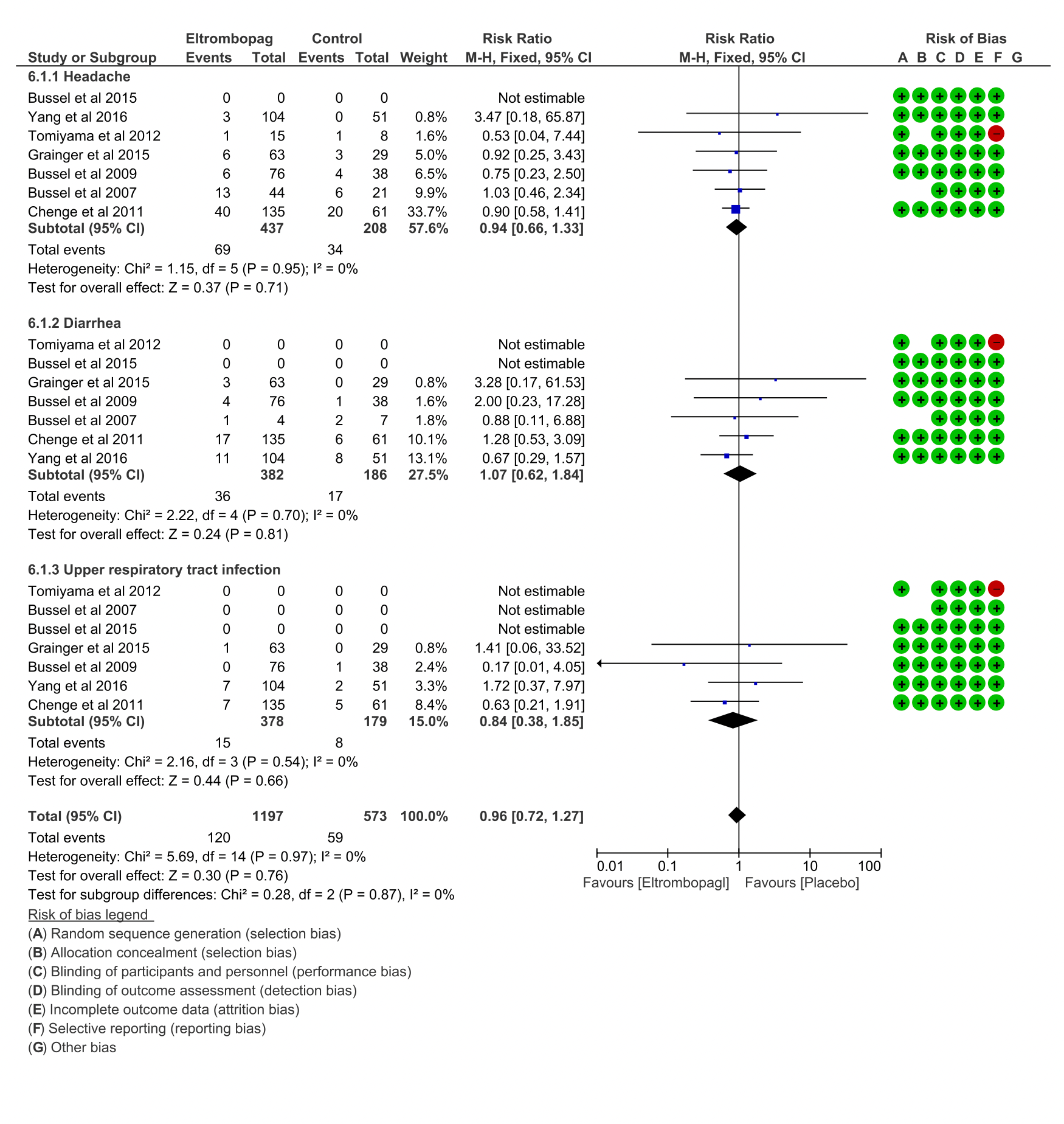
Results: Seven randomized controlled trials (N=766 patients) were included in the final analysis. Overall platelet response was significantly higher in the Eltrombopag group than in placebo (RR=3.90; 95%CI [2.89-5.25];P<0.00001) showing mild heterogeneity (I2=45%). Incidences of significant bleeding events in Eltrombopaggroup (World Health Organization [WHO] grades II-IV) (RR=0.63; 95% CI: [0.47-0.85]; P=0.003) showed lowerheterogeneity (I2=18%) in comparison to placebo group. Cases of use of rescue medications in Eltrombopag group compared to placebo group (RR=0.40; 95% CI: [0.29- 0.55]; P<0.00001) in all considered studies showed low heterogeneity (I2=41 %; P=0.16). Incidences of any bleeding in Eltrombopag group compared to placebo group(RR=0.77; 95% CI: [0.70-0.86]; P<0.00001; I2=65%) showed substantial heterogeneity. Finally, subgroup analysis of Eltrombopag efficiency revealed significant difference in frequency of bleeding cases between adults (RR=0.84)and children (RR=0.51); (P=0.005).
Conclusion: This systematic review presents class one evidence suggesting Eltrombopag as safe and effective drug for therapy of both children and adult patients with ITP.
Graphical Abstract
Keywords:
Eltrombopag, immune thrombocytopenia, safety and efficacy, thrombopoietin agonists, meta-analysisReferences
Agarwal N, Mangla A (2021) Thrombopoietin receptor agonist for treatment of immune thrombocytopenia in pregnancy: A narrative review. Therapeutic Advances in Hematology 12: 20406207211001140. https://doi.org/10.1177/20406207211001139 [PubMed] [PMC]
Ahmed M, Yassin MA, Abdelmahmuod E (2020) Steroid-refractory chronic idiopathic thrombocytopenic purpura responding to combination therapy with eltrombopag and rituximab. Cureus 12(9): e10305. https://doi.org/10.7759/cureus.10305 [PubMed] [PMC]
Bhat FA, Advani J, Khan AA, Mohan S, Pal A, Gowda H, Chakrabarti P, Keshava Prasad TS, Chatterjee A (2018) A network map of thrombopoietin signaling. Journal of Cell Communication and Signaling, 12(4): 737–743. https://doi.org/10.1007/s12079-018-0480-4 [PubMed] [PMC]
Blickstein D (2019) Treatment of immune thrombocytopenic purpura in adults: Update. Harefuah 158(3): 196–199. [in Hebrew] [PubMed]
Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, Kloczko J, Hassani H, Mayer B, Stone NL, Arning M, Provan D, Jenkins JM (2007) Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. New England Journal of Medicine 357(22): 2237–2247. https://doi.org/10.1056/NEJMoa073275 [PubMed]
Bussel JB, de Miguel PG, Despotovic JM, Grainger JD, Sevilla J, Blanchette VS, Krishnamurti L, Connor P, David M, Boayue KB, Matthews DC, Lambert MP, Marcello LM, Iyengar M, Chan GW, Chagin KD, Theodore D, Bailey CK, Bakshi KK (2015) Eltrombopag for the treatment of children with persistent and chronic immune thrombocytopenia (PETIT): A randomised, multicentre, placebo-controlled study. The Lancet Haematology 2(8): e315–e325. https://doi.org/https://doi.org/10.1016/S2352-3026(15)00114-3 [PubMed]
Bussel JB, Provan D, Shamsi T, Cheng G, Psaila B, Kovaleva L, Salama A, Jenkins JM, Roychowdhury D, Mayer B, Stone N, Arning M (2009) Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: A randomised, double-blind, placebo-controlled trial. The Lancet 373(9664): 641–648. https://doi.org/10.1016/S0140-6736(09)60402-5 [PubMed]
Cheng G, Saleh MN, Marcher C, Vasey S, Mayer B, Aivado M, Arning M, Stone NL, Bussel JB (2011) Eltrombopag for management of chronic immune thrombocytopenia (RAISE): A 6-month, randomised, phase 3 study. The Lancet 377(9763): 393–402. https://doi.org/https://doi.org/10.1016/S0140-6736(10)60959-2 [PubMed]
Etminan M, Collins GS, Mansournia MA (2020) Using causal diagrams to improve the design and interpretation of medical research. Chest 158(1): S21–S28. https://doi.org/10.1016/j.chest.2020.03.011 [PubMed]
Farrah K, Young K, Tunis MC, Zhao L (2019) Risk of bias tools in systematic reviews of health interventions: An analysis of PROSPERO-registered protocols. Systematic Reviews 8(1): 280. https://doi.org/10.1186/s13643-019-1172-8[PubMed] [PMC]
Furuya-Kanamori L, Barendregt JJ, Doi SAR (2018) A new improved graphical and quantitative method for detecting bias in meta-analysis. International Journal of Evidence-Based Healthcare 16(4): 195–203. https://doi.org/10.1097/XEB.0000000000000141 [PubMed]
Grainger JD, Locatelli F, Chotsampancharoen T, Donyush E, Pongtanakul B, Komvilaisak P, Sosothikul D, Drelichman G, Sirachainan N, Holzhauer S, Lebedev V, Lemons R, Pospisilova D, Ramenghi U, Bussel JB, Bakshi KK, Iyengar M, Chan GW, Chagin KD, Theodore D, Marcello LM, Bailey CK (2015) Eltrombopag for children with chronic immune thrombocytopenia (PETIT2): A randomised, multicentre, placebo-controlled trial. The Lancet 386(10004): 1649–1658. https://doi.org/https://doi.org/10.1016/S0140-6736(15)61107-2 [PubMed]
Koletsi D, Fleming PS, Michelaki I, Pandis N (2018) Heterogeneity in Cochrane and non-Cochrane meta-analyses in orthodontics. Journal of Dentistry 74: 90–94. https://doi.org/10.1016/j.jdent.2018.05.003 [PubMed]
Levchenkova OS, Vorobieva VV, Novikov VE, Legonkova TI, Volkova EN (2023) Thrombopoietin receptor agonists in pharmacotherapy of pediatric immune thrombocytopenia. Research Results in Pharmacology 9(1): 49–59. https://doi.org/10.18413/rrpharmacology.9.10011
Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N, Cuker A, Despotovic JM, George JN, Grace RF, Kühne T, Kuter DJ, Lim W, McCrae KR, Pruitt B, Shimanek H, Vesely SK (2019) American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Advances 3(23): 3829–3866. https://doi.org/10.1182/bloodadvances.2019000966 [PubMed] [PMC]
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Journal of Clinical Epidemiology 134: 178–189. https://doi.org/10.1016/j.jclinepi.2021.03.001 [PubMed]
Tomiyama Y, Miyakawa Y, Okamoto S, Katsutani S, Kimura A, Okoshi Y, Ninomiya H, Kosugi H, Nomura S, Ozaki K, Ikeda Y, Hattori T, Katsura K, Kanakura Y (2012) A lower starting dose of eltrombopag is efficacious in Japanese patients with previously treated chronic immune thrombocytopenia. Journal of Thrombosis and Haemostasis 10(5): 799–806. https://doi.org/10.1111/j.1538-7836.2012.04695.x [PubMed]
VasudevaVasudevan Nampoothiri R, Kumar R (2020) Eltrombopag: Role in cytopenias following hematopoietic stem cell transplantation. Indian Journal of Hematology & Blood Transfusion 36(2): 238–245. https://doi.org/10.1007/s12288-019-01194-7 [PubMed] [PMC]
Yang R, Li J, Jin J, Huang M, Yu Z, Xu X, Zhang X, Hou M (2017) Multicentre, randomised phase III study of the efficacy and safety of eltrombopag in Chinese patients with chronic immune thrombocytopenia. British Journal of Haematology 176(1): 101–110. https://doi.org/10.1111/bjh.14380 [PubMed]
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Research Results in Pharmacology

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

