Method of detection of dexamethasone in biological tissues and its application to assess the local kinetics of this drug
DOI:
https://doi.org/10.18413/rrpharmacology.9.10045Abstract
Introduction: The study of the pharmacokinetics of glucocorticosteroids is often required to solve fundamental and applied tasks of pharmacology. HPLC methods based on ultraviolet detection are attractive due to their availability, but their sensitivity is low enough to study in vivo kinetics. In this study, we propose a method for the determination of dexamethasone in biological objects, based on the use of HPLC with UV detection and having sufficient sensitivity to determine the drug in biological media (blood and periarticular tissues).
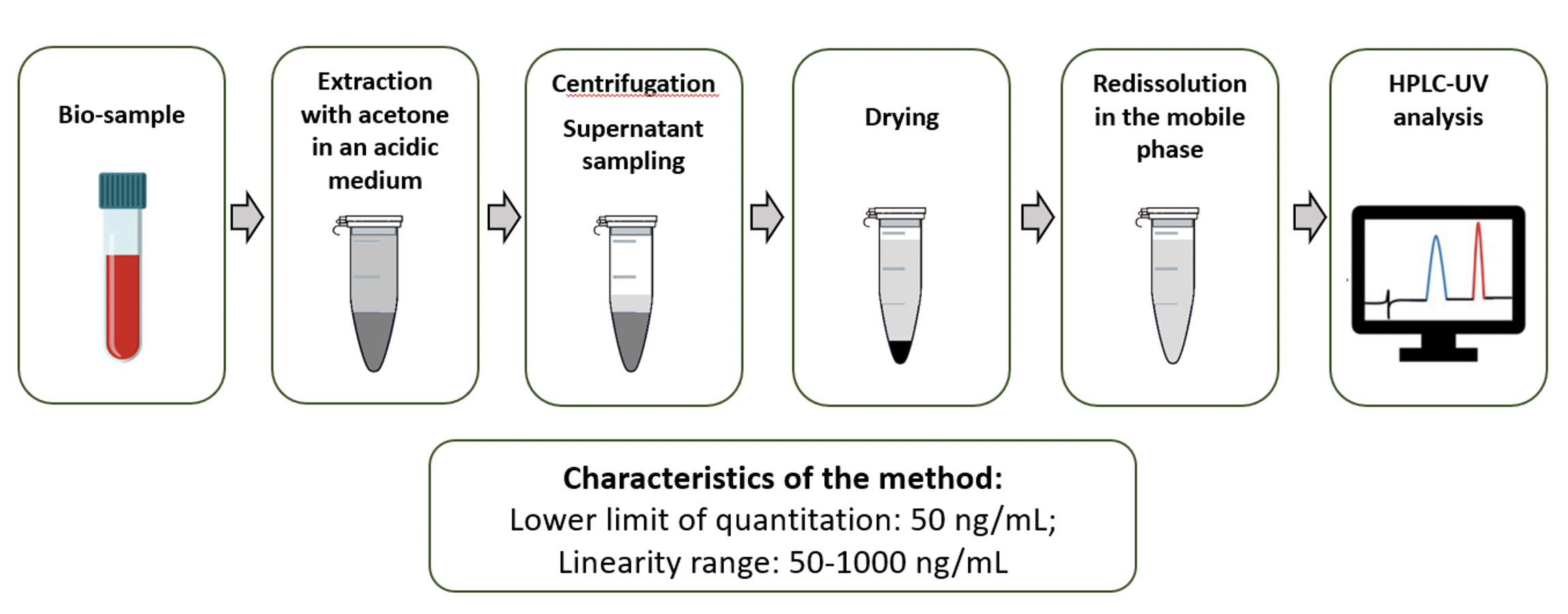
Materials and methods: Extraction of dexamethasone from biosamples was carried out by liquid-liquid extraction with acetone in an acidic medium using atenolol as an internal standard. The analysis was carried out on a Kromasil-100 C18 column. A mixture of methanol with phosphate buffer in the ratio 50÷50, pH=5.6 was used as the mobile phase. Detector - UV, wavelength - 254 nm. The LLOQ of the method was 50 ng/mL; the calibration curve demonstrated linearity in the con-centration range of 50-1000 ng/mL.The method was used to detect the medicinal product in peri-synovial tissues of rats with an autoimmune arthritis model.
Results: This study demonstrated that intraarticular injection of the liposomal form of dexamethasone, compared with its water-soluble form, allows maintaining the active concentration of the product in the joint and periarticular tissues for a longer time, which creates prerequisites for enhancing its therapeutic effect.
Conclusion: The proposed method provides a sensitive and specific approach for measuring dexamethasone in biological samples, such as blood and periarticular tissues. Preliminary findings indicate that the liposomal form of dexamethasone may exhibit better pharmacokinetic properties than the water-soluble form, which could lead to improved therapeutic outcomes.
Graphical Abstract
Keywords:
intraarticular injection, dexamethasone, liposomes, HPLC-UV detection, pharmacokineticsReferences
Badokin VV (2013) Locomotor therapy with extended-release crystalline glucocorticoids. Neurology, Neuropsychiatry, Psychosomatics [Nevrologiya, Neiropsikhiatriya, Psikhosomatika] 2: 88–92. https://doi.org/10.14412/2074-2711-2013-2420 [in Russian]
Brugnera M, Vicario-de-la-Torre M, Andrés-Guerrero V, Bravo-Osuna I, Molina-Martínez IT, Herrero-Vanrell R (2022) Validation of a rapid and easy-to-apply method to simultaneously quantify co-loaded dexamethasone and melatonin PLGA microspheres by HPLC-UV: Encapsulation efficiency and in vitro release. Pharmaceutics 14(2): 288. https://doi.org/10.3390/pharmaceutics14020288
Chen T, Zhang B, Lin Y, Pan J, Zhao X-X, Ren S-X (2019) Protective effects of prunasin a against the differentiation of osteoclasts and destruction of cartilage via the receptor acti-vator of nuclear factor-kappa-Β Ligand/mitogen-activated protein kinase/osteoprotegerin pathway in a rat model of arthritis. Pharmacology 104(5-6): 216–225. https://doi.org/10.1159/000502537 [PubMed]
Duarah S, Sharma M, Wen J (2021) Rapid and simultaneous determination of dexamethasone and dexamethasone sodium phosphate using HPLC-UV: Application in microneedle-assisted skin permeation and deposition studies. Journal of Chromatography B 1170: 122609. https://doi.org/10.1016/j.jchromb.2021.122609 [PubMed]
Earp JC, Pyszczynski NA, Molano DS, Jusko WJ (2008) Pharmacokinetics of dexamethasone in a rat model of rheumatoid arthritis. Biopharmaceutics & Drug Disposition 29(6): 366–372. https://doi.org/10.1002/bdd.626[PubMed] [PMC]
Jóhannesson G, Moya-Ortega MD, Ásgrímsdóttir GM, Lund SH, Thorsteinsdóttir M, Loftsson T, Stefánsson E (2014) Kinetics of γ -cyclodextrin nanoparticle suspension eye drops in tear fluid. Acta Ophthalmologica 92: 550–556. https://doi.org/10.1111/aos.12334 [PubMed]
Kulikov OA, Zaborovskiy AV, Unina DV, Gurevich KG, Tararina LA, Ageev VP, Shlyapkina VI, Pyataev NA, Mulyar AG, Andreev DN (2021) Evaluation of the efficacy of intraarticular in-jection of liposomal dexamethasone in a rheumatoid arthritis model in rats. Journal of Pharmaceutical Chemistry 55: 37–41. https://doi.org/10.30906/0023-1134-2021-55-5-37-41
Li L, Ma P, Wei J, Qian K, Tao L (2013) LC-ESI-MS method for the determination of dexame-thasone acetate in skin of nude mouse. Journal of Chromatography B 933: 44–49. https://doi.org/10.1016/j.jchromb.2013.06.024 [PubMed]
Mironov AN, Petrov VI, Merkulov VA (2013) Guidelines for the expert examination of drug products. Grif and K, Moscow, 328 pp. [in Russian]
Prieto E, Vispe E, Otín-Mallada S, Garcia-Martin E, Polo-Llorens V, Fraile JM, Pablo LE, Mayoral JA (2017) Determination of three corticosteroids in the biologic matrix of vitreous humor by HPLC-tandem mass spectrometry: Method development and validation. Current Eye Research 42(2): 244–251. https://doi.org/10.1080/02713683.2016.1183795 [PubMed]
Raguzin EV, Yudin MA, Glushenko DD, Vengerovich NG, Raguzina OG, Pechurina TB, Shefer TV, Ivanov IM (2022) Analysis and evaluation of modern approaches to the development of drugs using micro- and nanotechnologies. Russian Biomedical Bulletin named after academician I.P. Pavlov 30(3): 397–410. https://doi.org/10.17816/PAVLOVJ104787 [in Russian]
Song D, Sun L, DuBois DC, Almon RR, Meng S, Jusko WJ (2020) Physiologically based pharmacokinetics of dexamethasone in rats. Drug Metabolism and Disposition 48: 811–818. https://doi.org/10.1124/dmd.120.091017 [PubMed] [PMC]
Su C, Chen Y, Chen Y, Zhou Y, Li L, Lu Q, Liu H, Luo X, Zhu J (2019) Effect of electroacupunc-ture at the ST36 and GB39 acupoints on apoptosis by regulating the p53 signaling pathway in adjuvant arthritis rats. Molecular Medicine Reports 20(5): 4101–4110. https://doi.org/10.3892/mmr.2019.10674 [PubMed] [PMC]
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Дина В. Юнина, Валентин П. Агеев, Андрей В. Заборовский, Лариса А. Тарарина, Сергей В. Царегородцев, Александр В. Кокорев, Дмитрий Н. Андреев , Александра Е. Пьянзина , Василиса И. Шляпкина , Николай А. Пятаев, Олег А. Куликов

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

