Polyelectrolyte Microcapsules as a Tool to Enhance Photosensitizing Effect of Chlorin E6
DOI:
https://doi.org/10.18413/rrpharmacology.9.10055Abstract
Introduction: Photodynamic therapy is a promising method of tumors treatment using photosensitizers and light of a certain wavelength. PS modification improves and enhances the phototoxic effect with decreased dark cytotoxicity.
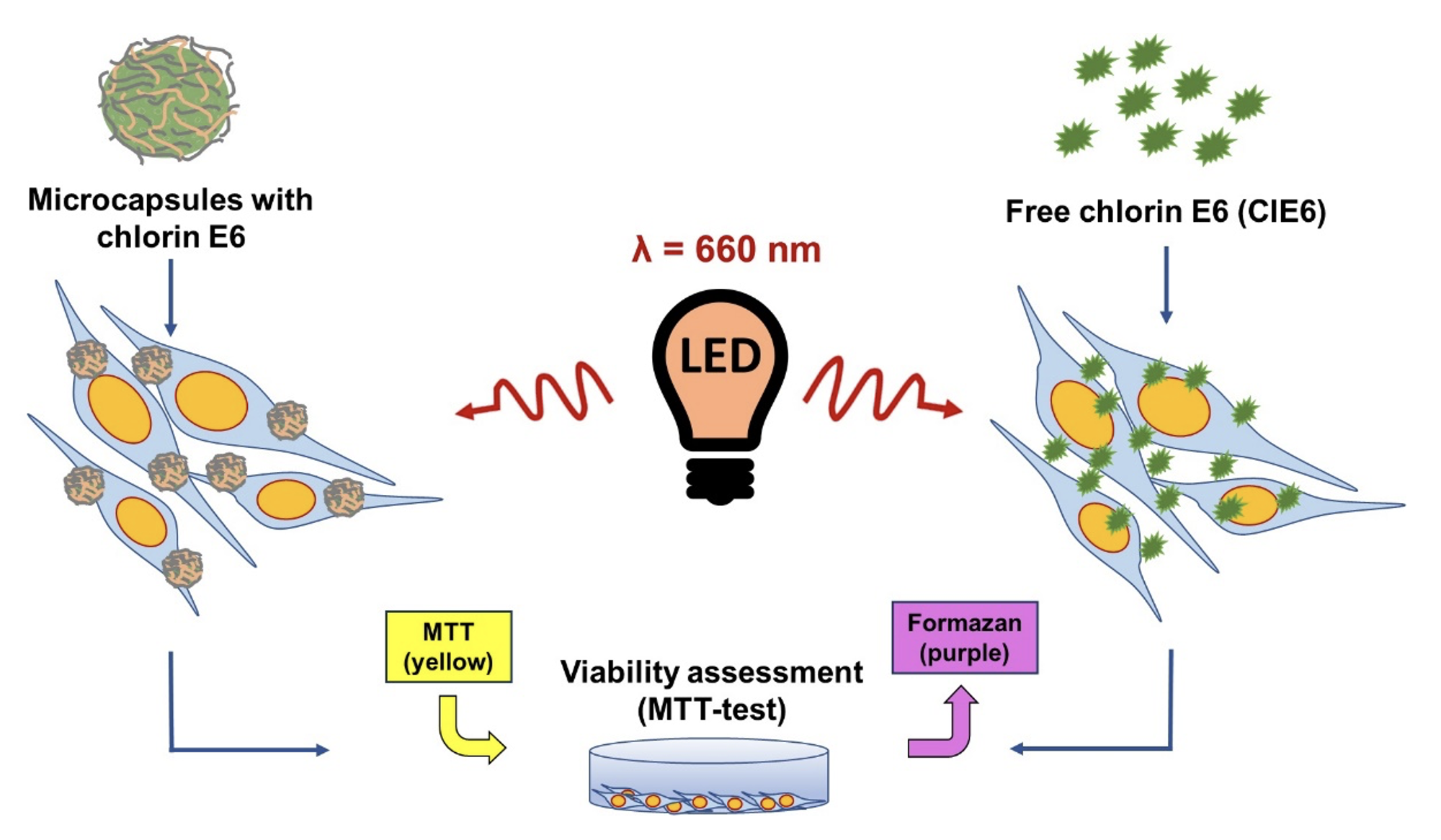
Materials and Methods: We compared the photosensitizing effect of polyelectrolyte microcapsules with chlorin E6 (ClE6) and free ClE6 at equivalent concentrations on murine fibroblast culture L929 using in vitro tests. Microcapsules were prepared layer by layer, sequentially depositing oppositely charged polyelectrolytes onto spherical CaCO3 particles. Cellular uptake of capsules was assessed using confocal microscopy. MTT test was used for a study of cell viability, and the relative amount of ROS was determined by the fluorescent method.
Results: Microcapsules with ClE6 (in all tested concentrations) after exposure to red light (660 nm) reduced cell viability from 20% to 5%, while these capsules did not have dark cytotoxicity. Free ClE6 at the same concentrations as in the capsules after irradiation reduced viability from 65% to 35%. The level of ROS in the group of cells with capsules was 2 times higher compared to the group with CLE6.
Discussion: The most probable mechanism of toxicity increase is creation of a higher ROS concentration and effect localization in the area of microcapsule interaction with the cell membrane. ROS production activation may stem from capsules providing a higher local PS concentration in the cell or nearby than the drug’s free form.
Conclusion: The inclusion of chlorin E6 in polymer capsules reduced dark toxicity and increased the photosensitizing effect compared to the free form of ClE6.
Graphical Abstract
Keywords:
polyelectrolyte microcapsules, photodynamic therapy, chlorin E6, phototoxicity, ROS – reactive oxygen speciesReferences
Bacellar IOL, Tsubone TM, Pavani C, Baptista MS (2015) Photodynamic efficiency: from molecular photochemistry to cell death. International Journal of Molecular Sciences 16(9): 20523–20559. https://doi.org/10.3390/ijms160920523 [PubMed] [PMC]
Bisland SK, Singh D, Gariépy J (1999) Potentiation of chlorin e6 photodynamic activity in vitro with peptide-based intracellular vehicles. Bioconjugate Chemistry 10(6): 982–992. https://doi.org/10.1021/bc990020u [PubMed]
Casas A (2020) Clinical uses of 5-aminolaevulinic acid in photodynamic treatment and photodetection of cancer: A review. Cancer Letters 490: 165–173. https://doi.org/10.1016/j.canlet.2020.06.008 [PubMed]
Castano AP, Demidova TN, Hamblin MR (2005) Mechanisms in photodynamic therapy: part two-cellular signaling, cell metabolism and modes of cell death. Photodiagnosis and Photodynamic Therapy 2(1): 1–23. https://doi.org/10.1016/S1572-1000(05)00030-X [PubMed] [PMC]
Chen J, Fan T, Xie Z, Zeng Q, Xue P, Zheng T, Chen Y, Luo X, Zhang H (2020) Advances in nanomaterials for photodynamic therapy applications: Status and challenges. Biomaterials 237: 119827. https://doi.org/10.1016/j.biomaterials.2020.119827 [PubMed]
De Geest BG, Vandenbroucke RE, Guenther AM, Sukhorukov GB, Hennink WE, Sanders NN, Demeester J, De Smedt SC (2006) Intracellularly degradable polyelectrolyte microcapsules. Advanced Materials 18: 1005–1009. https://doi.org/10.1002/adma.200502128
Donohoe C, Senge MO, Arnaut LG, Gomes-da-Silva LC (2019) Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochimica et Biophysica Acta. Reviews on Cancer 1872(2): 188308. https://doi.org/10.1016/j.bbcan.2019.07.003 [PubMed]
Duan X, Chen B, Cui Y, Zhou L, Wu C, Yang Z, Wen Y, Miao X, Li Q, Xiong L, He J (2018) Ready player one? Autophagy shapes resistance to photodynamic therapy in cancers. Apoptosis 23(11-12): 587–606. https://doi.org/10.1007/s10495-018-1489-0 [PubMed]
Javier AM, Kreft O, Semmling M, Kempter S, Skirtach AG, Bruns OT, Pino P del, Bedard MF, Rädler J, Käs J, Plank C, Sukhorukov GB, Parak WJ (2008) Uptake of colloidal polyelectrolyte-coated particles and polyelectrolyte multilayer capsules by living cells. Advanced Materials 20(22): 4281–4287. https://doi.org/10.1002/adma.200703190
Juzeniene A (2009) Chlorin e6-based photosensitizers for photodynamic therapy and photodiagnosis. Photodiagnosis and Photodynamic Therapy 6(2): 94–96. https://doi.org/10.1016/j.pdpdt.2009.06.001 [PubMed]
Kessel D (1977) Effects of photoactivated porphyrins at the cell surface of leukemia L1210 cells. Biochemistry 16(15): 3443–3449. https://doi.org/10.1021/bi00634a023 [PubMed] [PMC]
Lanza RP, Langer R, Vacanti J (2000) Principles of Tissue Engineering. Academic Press, New York, USA, 995 pp. https://doi.org/10.1016/B978-0-12-436630-5.X5000-4
McGahon AJ, Martin SJ, Bissonnette RP, Mahboubi A, Shi Y, Mogil RJ, Nishioka WK, Green DR (1995) The end of the (cell) line: methods for the study of apoptosis in vitro. Methods in Cell Biology 46: 153–185. https://doi.org/10.1016/s0091-679x(08)61929-9 [PubMed]
Ming L, Cheng K, Chen Y, Yang R, Chen D (2021) Enhancement of tumor lethality of ROS in photodynamic therapy. Cancer Medicine 10(1): 257–268. https://doi.org/10.1002/cam4.3592 [PubMed] [PMC]
Moan J (1990) On the diffusion length of singlet oxygen in cells and tissues. Journal of Photochemistry and Photobiology B: Biology 6(3): 343–344. https://doi.org/10.1016/1011-1344(90)85104-5
Moiseeva N, Eroshenko D, Laletina L, Rybalkina E, Susova O, Karamysheva A, Tolmacheva I, Nazarov M, Grishko V (2023) The molecular mechanisms of oleanane aldehyde-β-enone cytotoxicity against doxorubicin-resistant cancer cells. Biology 12(3): 415. https://doi.org/10.3390/biology12030415 [PubMed] [PMC]
Rejman J, Oberle V, Zuhorn IS, Hoekstra D (2004) Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochemical Journal 377(Pt 1): 159–169. https://doi.org/10.1042/bj20031253 [PubMed] [PMC]
Rosin FCP, Teixeira MG, Pelissari C, Corrêa L (2018) Resistance of oral cancer cells to 5-ALA-mediated photodynamic therapy. Journal of Cellular Biochemistry 119(4): 3554–3562. https://doi.org/10.1002/jcb.26541 [PubMed]
Soleymani M, Velashjerdi M, Shaterabadi Z, Barati A (2020) One-pot preparation of hyaluronic acid-coated iron oxide nanoparticles for magnetic hyperthermia therapy and targeting CD44-overexpressing cancer cells. Carbohydrate Polymers 237: 116130. https://doi.org/10.1016/j.carbpol.2020.116130 [PubMed]
Spikes JD (1984) Photobiology of porphyrins. Progress in Clinical and Biological Research 170: 19–39. [PubMed]
Volodkin DV, Petrov AI, Prevot M, Sukhorukov GB (2004) Matrix polyelectrolyte microcapsules: new system for macromolecule encapsulation. Langmuir 20(8): 3398–3406. https://doi.org/10.1021/la036177z [PubMed]
Xu D, Baidya A, Deng K, Li Y-S, Wu B, Xu H-B (2021) Multifunctional nanoparticle PEG‑Ce6‑Gd for MRI‑guided photodynamic therapy. Oncology Reports 45: 547–556. https://doi.org/10.3892/or.2020.7871 [PubMed] [PMC]
Ye T, Chen T, Jiang B, Yang L, Liu X, Chen B, Zou Y, Yu B (2020) 5-aminolevulinic acid photodynamic therapy inhibits invasion and metastasis of SCL-1 cells probably via MTSS1 and p63 gene related pathways. Photodiagnosis and Photodynamic Therapy 32: 102039. https://doi.org/10.1016/j.pdpdt.2020.102039 [PubMed]
Yin T, Huang P, Gao G, Shapter JG, Shen Y, Sun R, Yue C, Zhang C, Liu Y, Zhou S, Cui D (2016) Superparamagnetic Fe3O4-PEG2K-FA@Ce6 nanoprobes for in vivo dual-mode imaging and targeted photodynamic therapy. Scientific Reports 6: 36187. https://doi.org/10.1038/srep36187 [PubMed] [PMC]
Zharkov MN, Brodovskaya EP, Kulikov OA, Gromova EV, Ageev VP, Atanova AV, Kozyreva ZV, Tishin AM, Pyatakov AP, Pyataev NA, Sukhorukov GB (2021) Enhanced cytotoxicity caused by AC magnetic field for polymer microcapsules containing packed magnetic nanoparticles. Colloids and Surfaces. B, Biointerfaces 199: 111548. https://doi.org/10.1016/j.colsurfb.2020.111548 [PubMed]
Zhou J, Geng S, Ye W, Wang Q, Lou R, Yin Q, Du B, Yao H (2020) ROS-boosted photodynamic therapy against metastatic melanoma by inhibiting the activity of antioxidase and oxygen-producing nano-dopants. Pharmacological Research 158: 104885. https://doi.org/10.1016/j.phrs.2020.104885 [PubMed]
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Екатерина П. Бродовская , Лариса А. Тарарина, Михаил Н. Жарков , Ирина А. Хуторская , Денис Э. Якобсон, Амина Аль-хадж Аюб , Игорь В. Маев, Андрей В. Заборовский , Дина В. Юнина, Сергей В. Царегородцев, Глеб Б. Сухоруков, Николай А. Пятаев

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

