Proteins with the HAEE tetrapeptide motif as potential targets for beta-amyloid
DOI:
https://doi.org/10.18413/rrpharmacology.11.1046Abstract
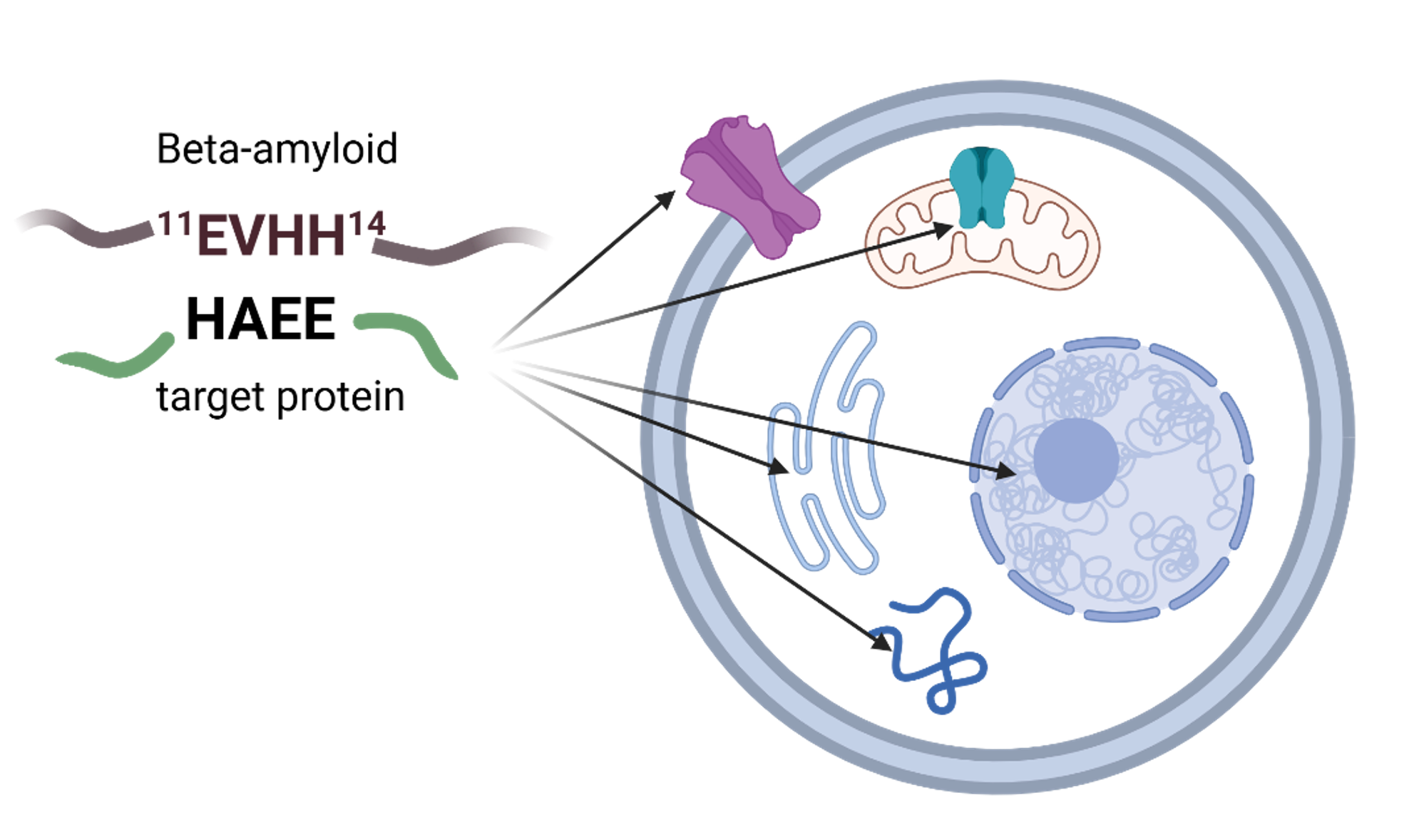
Introduction: Beta-amyloid (Aβ) is involved in numerous physiological and pathophysiological processes and is one of the key players in the pathogenesis of Alzheimer’s disease. Aβ interacts with the 35-HAEE-38 site of the α4 subunit of the α4β2 nicotinic acetylcholine receptor. The synthetic tetrapeptide HAEE effectively inhibits the aggregation of endogenous Aβ. HAEE specifically binds to the 11-EVHH-14 site of Aβ both in the absence and presence of zinc ions, leading to the formation of stable complexes. We hypothesized that the HAEE motif could represent a universal binding site for Aβ within the human proteome.
Materials and Methods: To test this hypothesis, a large-scale search for all amino acid sequences containing the HAEE motif in the human (Homo sapiens) proteome was performed using our in-house PepString server (http://pepstring.eimb.ru/). The conservation of the identified sites was analyzed across jawed vertebrates using BLAST. Protein localization and structural features were determined based on data from UniProt, PDB, and AlphaFold.
Results: We identified 85 proteins (including 200 isoforms) containing the HAEE motif. Of these, 26 proteins are membrane proteins, including receptors, ion channels, and transporters (e.g., CACNA1B, MRS2, SLC15A2), and 59 are intracellular proteins, mostly nuclear transcription factors (including 13 zinc finger proteins). Structural analysis revealed that the HAEE motif is often located within functionally important domains, such as cytoplasmic loops of transmembrane proteins or DNA-binding domains.
Conclusion: Given that Aβ acts as an extracellular ligand and can also penetrate various intracellular compartments, all identified proteins with the HAEE motif are considered potential physiological and pathophysiological targets for Aβ. The most promising candidates are proteins whose HAEE sites are structurally similar to that in α4β2-nAChR and/or coordinate zinc ions. These findings enhance our understanding of the molecular mechanisms of Aβ function and open new avenues for the search of therapeutic targets in AD.
Graphical Abstract
Keywords:
Alzheimer's disease, beta-amyloid, molecular targets, short linear motifs, bioinformatic analysis, proteome, HAEEReferences
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. Journal of Molecular Biology 215(3): 403–410. https://doi.org/10.1016/S0022-2836(05)80360-2 [PubMed]
Anwar MU, Sergeeva OA, Abrami L, Mesquita FS, Lukonin I, Amen T, Chuat A, Capolupo L, Liberali P, D’Angelo G, Goot FG van der (2022) ER-Golgi-localized proteins TMED2 and TMED10 control the formation of plasma membrane lipid nanodomains. Developmental Cell 57(19): 2334–2346.e8. https://doi.org/10.1016/j.devcel.2022.09.004 [PubMed]
Barykin EP, Garifulina AI, Tolstova AP, Anashkina AA, Adzhubei AA, Mezentsev YV, Shelukhina IV, Kozin SA, Tsetlin VI, Makarov AA (2020) Tetrapeptide Ac-HAEE-NH2 protects α4β2 nAChR from inhibition by Aβ. International Journal of Molecular Sciences 21: 6272. https://doi.org/10.3390/ijms21176272 [PubMed] [PMC]
Brothers HM, Gosztyla ML, Robinson SR (2018) The physiological roles of amyloid-β peptide hint at new ways to treat Alzheimer’s disease. Frontiers in Aging Neuroscience 10: 118. https://doi.org/10.3389/fnagi.2018.00118 [PubMed] [PMC]
Cadoni MPL, Coradduzza D, Congiargiu A, Sedda S, Zinellu A, Medici S, Nivoli AM, Carru C (2024) Platelet dynamics in neurodegenerative disorders: investigating the role of platelets in neurological pathology. Journal of Clinical Medicine 13(7): 2102. https://doi.org/10.3390/jcm13072102 [PubMed] [PMC]
Chen G-F, Xu T-H, Yan Y, Zhou Y-R, Jiang Y, Melcher K, Xu HE (2017) Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacologica Sinica 38(9): 1205–1235. https://doi.org/10.1038/aps.2017.28 [PubMed] [PMC]
Chertkova RV, Brazhe NA, Bryantseva TV, Nekrasov AN, Dolgikh DA, Yusipovich AI, Sosnovtseva O, Maksimov GV, Rubin AB, Kirpichnikov MP (2017) New insight into the mechanism of mitochondrial cytochrome c function. PLoS ONE 12(5): e0178280.https://doi.org/10.1371/journal.pone.0178280 [PubMed] [PMC]
Cohen SIA, Linse S, Luheshi LM, Hellstrand E, White DA, Rajah L, Otzen DE, Vendruscolo M, Dobson CM, Knowles TPJ (2013) Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism. Proceedings of the National Academy of Sciences 110(24): 9758–9763. https://doi.org/10.1073/pnas.1218402110 [PubMed] [PMC]
Gao S, Yao X, Yan N (2021) Structure of human Cav2.2 channel blocked by the painkiller ziconotide. Nature 596(7870): 143–147. https://doi.org/10.1038/s41586-021-03699-6[PubMed] [PMC]
Golde TE, DeKosky ST, Galasko D (2018) Alzheimer’s disease: The right drug, the right time. Science (New York, N.Y.) 362: 1250–1251. https://doi.org/10.1126/science.aau0437
Han HJ, Tokino T, Nakamura Y (1998) CSR, a scavenger receptor-like protein with a protective role against cellular damage caused by UV irradiation and oxidative stress. Human Molecular Genetics 7(6): 1039–1046. https://doi.org/10.1093/hmg/7.6.1039 [PubMed]
Hartmann T (1999) Intracellular biology of Alzheimer’s disease amyloid beta peptide. European Archives of Psychiatry and Clinical Neuroscience 249(6): 291–298. https://doi.org/10.1007/s004060050102 [PubMed]
Jeong H, Shin H, Hong S, Kim Y (2022) Physiological roles of monomeric amyloid-β and implications for Alzheimer’s disease therapeutics. Experimental Neurobiology 31(2): 65–88. https://doi.org/10.5607/en22004 [PubMed] [PMC]
Jones N, Hardy WR, Friese MB, Jorgensen C, Smith MJ, Woody NM, Burden SJ, Pawson T (2007) Analysis of a Shc family adaptor protein, ShcD/Shc4, that associates with muscle-specific kinase. Molecular and Cellular Biology 27(13): 4759–4773. https://doi.org/10.1128/MCB.00184-07 [PubMed] [PMC]
Khaminets A, Heinrich T, Mari M, Grumati P, Huebner AK, Akutsu M, Liebmann L, Stolz A, Nietzsche S, Koch N, Mauthe M, Katona I, Qualmann B, Weis J, Reggiori F, Kurth I, Hübner CA, Dikic I (2015) Regulation of endoplasmic reticulum turnover by selective autophagy. Nature 522(7556): 354–358. https://doi.org/10.1038/nature14498 [PubMed]
Kozin SA, Anashkina AA, Matsuga DG, Suvaan BS, Tumanyan VG, Mitkevich VA, Makarov AA (2025) PepString server as a tool to search for short amino acid subsequences: identification of potential amyloid-beta targets. Acta Naturae 17(3): 67–76. https://doi.org/10.32607/actanaturae.27630 [PubMed] [PMC]
Kurth I, Pamminger T, Hennings JC, Soehendra D, Huebner AK, Rotthier A, Baets J, Senderek J, Topaloglu H, Farrell SA, Nürnberg G, Nürnberg P, De Jonghe P, Gal A, Kaether C, Timmerman V, Hübner CA (2009) Mutations in FAM134B, encoding a newly identified Golgi protein, cause severe sensory and autonomic neuropathy. Nature Genetics 41(11): 1179–1181. https://doi.org/10.1038/ng.464 [PubMed]
Lai LTF, Balaraman J, Zhou F, Matthies D (2023) Cryo-EM structures of human magnesium channel MRS2 reveal gating and regulatory mechanisms. Nature Communications 14: 7207. https://doi.org/10.1038/s41467-023-42599-3 [PubMed] [PMC
Lang DH, Yeung CK, Peter RM, Ibarra C, Gasser R, Itagaki K, Philpot RM, Rettie AE (1998) Isoform specificity of trimethylamine N-oxygenation by human flavin-containing monooxygenase (FMO) and P450 enzymes: selective catalysis by FMO3. Biochemical Pharmacology 56(8): 1005–1012. https://doi.org/10.1016/s0006-2952(98)00218-4[PubMed]
Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet (London, England) 396(10248): 413–446. https://doi.org/10.1016/S0140-6736(20)30367-6 [PubMed] [PMC]
Long JM, Holtzman DM (2019) Alzheimer disease: An update on pathobiology and treatment strategies. Cell 179(2): 312–339. https://doi.org/10.1016/j.cell.2019.09.001 [PubMed] [PMC]
Mitkevich VA, Barykin EP, Eremina S, Pani B, Katkova-Zhukotskaya O, Polshakov VI, Adzhubei AA, Kozin SA, Mironov AS, Makarov AA, Nudler E (2023) Zn-dependent β-amyloid aggregation and its reversal by the tetrapeptide HAEE. Aging and Disease 14: 309–318. https://doi.org/10.14336/AD.2022.0827 [PubMed] [PMC]
Pagani L, Eckert A (2011) Amyloid‐Beta interaction with mitochondria. International Journal of Alzheimer’s Disease 2011: 925050. https://doi.org/10.4061/2011/925050 [PubMed] [PMC]
Piskacek M, Zotova L, Zsurka G, Schweyen RJ (2009) Conditional knockdown of hMRS2 results in loss of mitochondrial Mg+ uptake and cell death. Journal of Cellular and Molecular Medicine 13(4): 693–700. https://doi.org/10.1111/j.1582-4934.2008.00328.x [PubMed] [PMC]
Querfurth HW, LaFerla FM (2010) Mechanisms of disease. The New England Journal of Medicine. 362(4): 329–344. https://doi.org/10.1056/NEJMra0909142 [PubMed]
Rawden HC, Kokwaro GO, Ward SA, Edwards G (2000) Relative contribution of cytochromes P-450 and flavin-containing monoxygenases to the metabolism of albendazole by human liver microsomes. British Journal of Clinical Pharmacology 49(4): 313–322. https://doi.org/10.1046/j.1365-2125.2000.00170.x [PubMed] [PMC]
Roher AE, Esh CL, Kokjohn TA, Castaño EM, Van Vickle GD, Kalback WM, Patton RL, Luehrs DC, Daugs ID, Kuo Y-M, Emmerling MR, Soares H, Quinn JF, Kaye J, Connor DJ, Silverberg NB, Adler CH, Seward JD, Beach TG, Sabbagh MN (2009) Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 5(1): 18–29. https://doi.org/10.1016/j.jalz.2008.10.004 [PubMed] [PMC]
Surguchov A, Emamzadeh FN, Titova M, Surguchev AA (2023) Controversial properties of amyloidogenic proteins and peptides: new data in the COVID era. Biomedicines 11(4): 1215. https://doi.org/10.3390/biomedicines11041215 [PubMed] [PMC]
Veeravalli S, Phillips IR, Freire RT, Varshavi D, Everett JR, Shephard EA (2020) Flavin-containing monooxygenase 1 catalyzes the production of taurine from hypotaurine. Drug Metabolism and Disposition 48(5): 378–385. https://doi.org/10.1124/dmd.119.089995[PubMed]
Walker LC (2020) Aβ plaques. Free Neuropathology 1: 31. https://doi.org/10.17879/freeneuropathology-2020-3025 [PubMed] [PMC]
Wang J, Gu BJ, Masters CL, Wang Y-J (2017) A systemic view of Alzheimer disease – insights from amyloid-β metabolism beyond the brain. Nature Reviews. Neurology 13(10): 612–623. https://doi.org/10.1038/nrneurol.2017.111 [PubMed]
Zaru R, Orchard S, UniProt Consortium (2023) UniProt tools: BLAST, align, peptide search, and ID mapping. Current Protocols 3(3): e697. https://doi.org/10.1002/cpz1.697 [PubMed] [PMC]
Zsurka G, Gregán J, Schweyen RJ (2001) The human mitochondrial Mrs2 protein functionally substitutes for its yeast homologue, a candidate magnesium transporter. Genomics 72(2): 158–168. https://doi.org/10.1006/geno.2000.6407 [PubMed]
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Anashkina AA, Kozin SA, Korokin MV, Mitkevich VA

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

