Effect of a brain penetrant 5-HT7 receptor agonist LP-211 alone and in combination with gabapentin on cognitive deficits in insulin treated diabetic neuropathic rats
DOI:
https://doi.org/10.18413/rrpharmacology.11.579Abstract
Introduction: Cognitive deficits were found to be more pronounced in diabetic neuropathic pain (DNP) patients, with limited treatment options. This study investigates the effects of 5-HT7 agonist (LP-211), alone and in conjunction with gabapentin, on cognitive deficits in insulin-treated DNP (IDNP) rats.
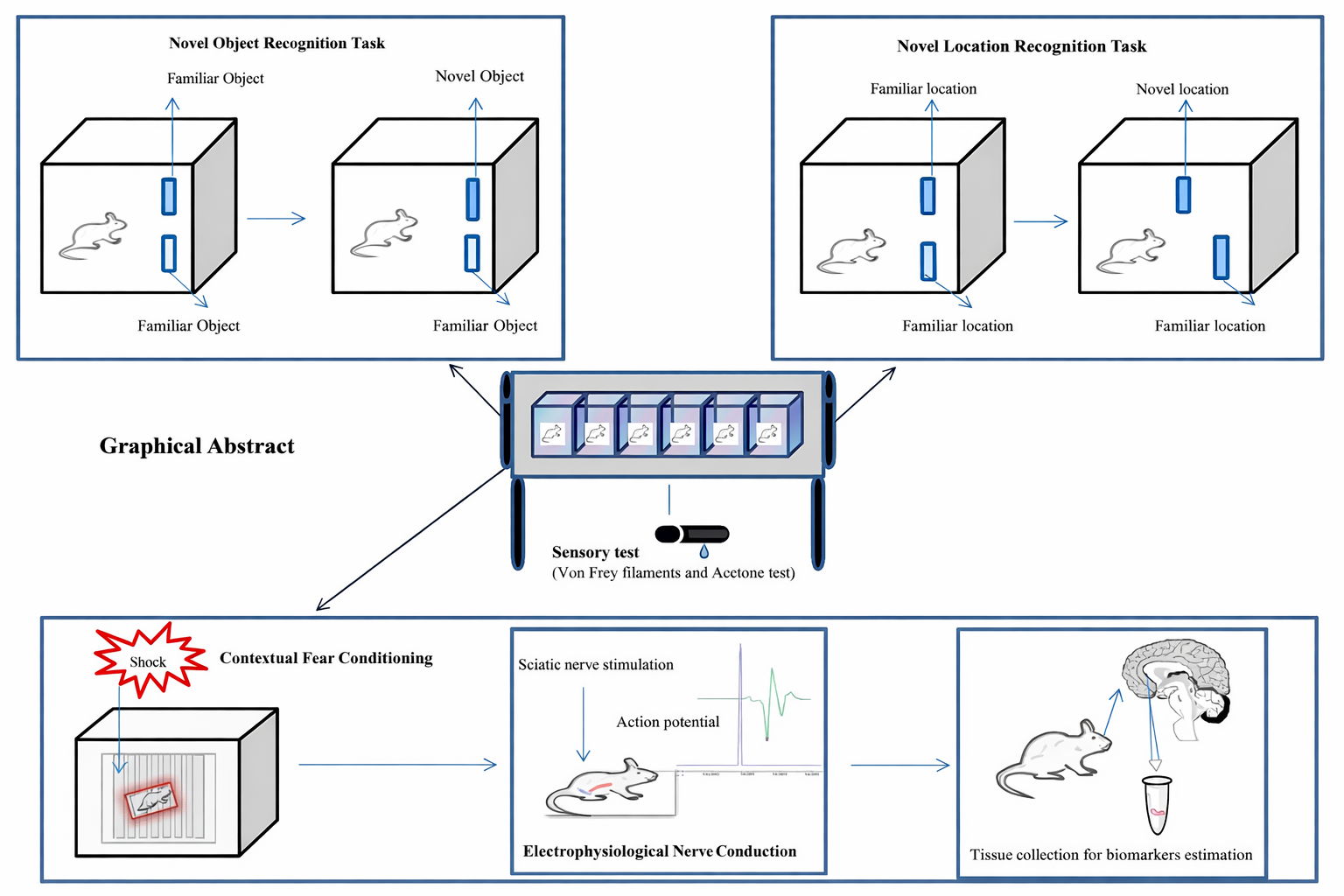
Materials and Methods: Diabetes was induced in male rats (N=71) using streptozotocin (50 mg/kg, intraperitoneally). DNP was assessed using Von Frey and acetone tests. Selected DNP rats received insulin (2 IU/kg, subcutaneously) daily. Treatments included LP-211 (3 mg/kg), gabapentin (10 mg/kg), combination (LP-211 + gabapentin), and donepezil(1mg/kg), which were administered intraperitoneally. Cognitive performances were evaluated through novel object recognition, novel location recognition, and fear conditioning tasks. Biomarkers such as monoamines, brain-derived neurotrophic factor (BDNF), interleukin-1 beta (IL-1β), and nerve conduction velocity (NCV) were measured.
Results and Discussion: Insulin maintained blood glucose levels, but did not alleviate cognitive deficits. LP-211, 3 mg/kg,donepezil, 1 mg/kg and LP-211, 3 mg/kg + gabapentin, 10 mg/kg demonstrated a significant discriminative index between novel and familiar objects, as well as object locations. Only combination treatment showed significant retrieval of percentage freezing and regulated biomarkers like NCV, monoamines, BDNF, and IL-1β levels compared to IDNP vehicle group. Donepezil modulated monoamines and BDNF, especially, LP-211, 3 mg/kg and donepezil; 1 mg/kg alone did not enhance the freezing response in IDNP rats. Overall, combination treatment notably showed enhanced associative learning, recognition, and spatial memory capabilities.
Conclusion: Acute treatment with LP-211 (3 mg/kg) + gabapentin (10 mg/kg) effectively improved integrated nature of memory in IDNP rats.
Graphical Abstract
Keywords:
Diabetic neuropathic pain, cognition, monoamines, brain-derived neurotrophic factor, interleukin-1 beta, nerve conduction velocityReferences
Alba-Delgado C, Cebada-Aleu A, Mico JA, Berrocoso E (2016) Comorbid anxiety-like behavior and locus coeruleus impairment in diabetic peripheral neuropathy: A comparative study with the chronic constriction injury model. Progress in Neuropsychopharmacology and Biological Psychiatry 71: 45– https://doi.org/10.1016/j.pnpbp.2016.06.007 [PubMed]
American Diabetes Association (2009) Diagnosis and classification of diabetes mellitus. Diabetes Care Suppl 1(Suppl 1): S62– https://doi.org/10.2337/dc09-s062 [PubMed] [PMC]
Ansari MA, Al-Jarallah A, Babiker FA (2023) Impaired insulin signaling alters mediators of hippocampal synaptic dynamics/plasticity: A possible mechanism of hyperglycemia-induced cognitive impairment. Cells 12(13): 1728. https://doi.org/10.3390/cells12131728 [PubMed] [PMC]
Atef MM, El-Sayed NM, Ahmed AAM, Mostafa YM (2019) Donepezil improves neuropathy through activation of AMPK signaling pathway in streptozotocin-induced diabetic mice. Biochemical Pharmacology 159: 1– https://doi.org/10.1016/j.bcp.2018.11.006 [PubMed]
Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, Yirmiya R (2003) Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus 13(7): 826– https://doi.org/10.1002/hipo.10135 [PubMed]
Baluchnejadmojarad T, Kiasalari Z, Afshin-Majd S, Ghasemi Z, Roghani M (2017) S-allyl cysteine ameliorates cognitive deficits in streptozotocin-diabetic rats via suppression of oxidative stress, inflammation, and acetylcholinesterase. European Journal of Pharmacology 794: 69– https://doi.org/10.1016/j.ejphar.2016.11.033[PubMed]
Beaudet G, Bouet V, Jozet-Alves C, Schumann-Bard P, Dauphin F, Paizanis E, Boulouard M, Freret T (2015) Spatial memory deficit across aging: current insights of the role of 5-HT7 receptors. Frontiers in Behavioral Neuroscience 8: 448. https://doi.org/10.3389/fnbeh.2014.00448 [PubMed] [PMC]
Beaudet G, Paizanis E, Zoratto F, Lacivita E, Leopoldo M, Freret T, Laviola G, Boulouard M, Adriani W (2017) LP-211, a selective 5-HT7 receptor agonist, increases novelty-preference and promotes risk-prone behavior in rats. Synapse 71(12). https://doi.org/10.1002/syn.21995 [PubMed]
Beeri MS, Sonnen J (2016) Brain BDNF expression as a biomarker for cognitive reserve against Alzheimer disease progression. Neurology 86(8): 702– https://doi.org/10.1212/wnl.0000000000002389 [PubMed]
Bellush LL, Reid SG (1991) Altered behavior and neurochemistry during short-term insulin withdrawal in streptozocin-induced diabetic rats. Diabetes 40(2): 217– https://doi.org/10.2337/diab.40.2.217 [PubMed]
Cai Y, Li X, Zhou H, Zhou J (2022) The serotonergic system dysfunction in diabetes mellitus. Frontiers in Cellular Neuroscience 16: 899069. https://doi.org/10.3389/fncel.2022.899069 [PubMed] [PMC]
Carbone C, Adinolfi A, Cinque S, Lacivita E, Alleva E, Leopoldo M, Adriani W (2018) Activation of 5-HT7 receptor by administration of its selective agonist, LP-211, modifies explorative-curiosity behavior in rats in two paradigms which differ in visuospatial parameters. CNS Neuroscience and Therapeutics 24(8): 712– https://doi.org/10.1111/cns.12812 [PubMed] [PMC]
Chaves YC, Raymundi AM, Waltrick APF, de Souza Crippa JA, Stern CAJ, da Cunha JM, Zanoveli JM (2024) Cannabidiol modulates contextual fear memory consolidation in animals with experimentally induced type-1 diabetes mellitus. Acta Neuropsychiatrica 36(5): 276– https://doi.org/10.1017/neu.2023.13 [PubMed]
Chincholkar M (2018) Analgesic mechanisms of gabapentinoids and effects in experimental pain models: a narrative review. British Journal of Anaesthesia 120(6): 1315– https://doi.org/10.1016/j.bja.2018.02.066[PubMed]
Coray R, Quednow BB (2022) The role of serotonin in declarative memory: A systematic review of animal and human research. Neuroscience and Biobehavioral Reviews 139: 104729. https://doi.org/10.1016/j.neubiorev.2022.104729 [PubMed]
Costa L, Sardone LM, Bonaccorso CM, D'Antoni S, Spatuzza M, Gulisano W, Tropea MR, Puzzo D, Leopoldo M, Lacivita E, Catania MV, Ciranna L (2018) Activation of serotonin 5-HT7 receptors modulates hippocampal synaptic plasticity by stimulation of adenylate cyclases and rescues learning and behavior in a mouse model of fragile X syndrome. Frontiers in Molecular Neuroscience 11: 353. https://doi.org/10.3389/fnmol.2018.00353[PubMed] [PMC]
Cummings JL, Osse AML, Kinney JW, Cammann D, Chen J (2024) Alzheimer’s disease: Combination therapies and clinical trials for combination therapy development. CNS Drugs 38(8): 613– https://doi.org/10.1007/s40263-024-01103-1 [PubMed] [PMC]
D'Amico D, Gener T, de Lagrán MM, Sanchez-Vives MV, Santos M, Dierssen M (2017) Infralimbic neurotrophin-3 infusion rescues fear extinction impairment in a mouse model of pathological fear. Neuropsychopharmacology 42(2): 462– https://doi.org/10.1038/npp.2016.154 [PubMed] [PMC]
De Filippis B, Chiodi V, Adriani W, Lacivita E, Mallozzi C, Leopoldo M, Domenici MR, Fuso A, Laviola G (2015) Long-lasting beneficial effects of central serotonin receptor 7 stimulation in female mice modeling Rett syndrome. Frontiers in Behavioral Neuroscience 9: 86. https://doi.org/10.3389/fnbeh.2015.00086 [PubMed] [PMC]
Ebata-KogureN, Nozawa KM, Aya T, Tetsumi HagaY, Fujii K (2017) Clinical and economic burdens experienced by patients with painful diabetic peripheral neuropathy: An observational study using a Japanese claims database. PLoS One 12(10): e0187250. https://doi.org/10.1371/journal.pone.0187250 [PubMed] [PMC]
Fontanesi LB, Fazan FS, Dias FJ, Schiavoni MCL, Marques W Jr, Fazan VPS (2019) Sensory and motor conduction velocity in spontaneously hypertensive rats: sex and aging investigation. Frontiers in Systems Neuroscience 13: 62. https://doi.org/10.3389/fnsys.2019.00062 [PubMed] [PMC]
Gallo A, Pillet LE, Verpillot R (2021) New frontiers in Alzheimer’s disease diagnostic: Monoamines and their derivatives in biological fluids. Experimental Gerontology 152: 111452. https://doi.org/10.1016/j.exger.2021.111452 [PubMed]
Gáspár A, Hutka B, Ernyey AJ, Tajti BT, Varga BT, Zádori ZS, Gyertyán I (2022) Performance of the intracerebroventricularly injected streptozotocin Alzheimer’s disease model in a translationally relevant, aged and experienced rat population. Scientific Reports 12(1): 20247. https://doi.org/10.1038/s41598-022-24292-5[PubMed] [PMC]
González-Sanmiguel J, Burgos CF, Bascuñán D, Fernández-Pérez EJ, Riffo-Lepe N, Boopathi S, Fernández-Pérez A, Bobadilla-Azócar C, González W, Figueroa M, Vicente B, Aguayo LG (2020) Gabapentin inhibits multiple steps in the amyloid beta toxicity cascade. ACS Chemical Neuroscience 11(19): 3064– https://doi.org/10.1021/acschemneuro.0c00414 [PubMed]
Groeneveld O, Reijmer Y, Heinen R, Kuijf H, Koekkoek P, Janssen J, Rutten G, Kappelle L, Biessels G; COG-ID study group (2018) Brain imaging correlates of mild cognitive impairment and early dementia in patients with type 2 diabetes mellitus. Nutrition, Metabolism & Cardiovascular Diseases 28(12): 1253– https://doi.org/10.1016/j.numecd.2018.07.008 [PubMed]
Gui WS, Wei X, Mai CL, Murugan M, Wu LJ, Xin WJ, Zhou LJ, Liu XG (2016) Interleukin-1β overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Molecular Pain 12: 1744806916646784. https://doi.org/10.1177/1744806916646784 [PubMed] [PMC]
Gwathmey KG, Pearson KT (2019) Diagnosis and management of sensory polyneuropathy. British Medical Journal365: l1108. https://doi.org/10.1136/bmj.l1108
Hao S, Shi W, Liu W, Chen QY, Zhuo M (2023) Multiple modulatory roles of serotonin in chronic pain and injury-related anxiety. Frontiers in Synaptic Neuroscience 15: 1122381. https://doi.org/10.3389/fnsyn.2023.1122381[PubMed] [PMC]
Hong JSW, Atkinson LZ, Al-Juffali N, Awad A, Geddes JR, Tunbridge EM, Harrison PJ, Cipriani A (2022) Gabapentin and pregabalin in bipolar disorder, anxiety states, and insomnia: Systematic review, meta-analysis, and rationale. Molecular Psychiatry 27(3): 1339– https://doi.org/10.1038/s41380-021-01386-6 [PubMed] [PMC]
Huang W, Lin Z, Sun A, Deng J, Manyande A, Xiang H, Zhao GF, Hong Q (2023) The role of gut microbiota in diabetic peripheral neuropathy rats with cognitive dysfunction. Frontiers in Microbiology 14: 1156591. https://doi.org/10.3389/fmicb.2023.1156591 [PubMed] [PMC]
Hussein RA, Afifi AH, Soliman AAF, El Shahid ZA, Zoheir KMA, Mahmoud KM (2020) Neuroprotective activity of Ulmus pumila L. in Alzheimer’s disease in rats; role of neurotrophic factors. Heliyon 6(12): e05678. https://doi.org/10.1016/j.heliyon.2020.e05678 [PubMed] [PMC]
Hyllienmark L, Alstrand N, Jonsson B, Ludvigsson J, Cooray G, Wahlberg-Topp J (2013) Early electrophysiological abnormalities and clinical neuropathy: A prospective study in patients with type 1 diabetes. Diabetes Care 36(10): 3187– https://doi.org/10.2337/dc12-2226 [PubMed] [PMC]
Izquierdo I, Medina JH, Vianna MR, Izquierdo LA, Barros DM (1999) Separate mechanisms for short- and long-term memory. Behavioural Brain Research 103(1): 1– https://doi.org/10.1016/S0166-4328(99)00036-4[PubMed]
Jesus CHA, Scarante FF, Schreiber AK, Gasparin AT, Redivo DDB, Rosa ES, da Cunha JM (2022) Comparative study of cold hyperalgesia and mechanical allodynia in two animal models of neuropathic pain: Different etiologies and distinct pathophysiological mechanisms. Brazilian Journal of Pharmaceutical Sciences 58: e20637. https://doi.org/10.1590/s2175-97902022e20637
Jian WX, Zhang Z, Zhan JH, Chu SF, Peng Y, Zhao M, Wang Q, Chen NH (2020) Donepezil attenuates vascular dementia in rats through increasing BDNF induced by reducing HDAC6 nuclear translocation. Acta Pharmacologica Sinica 41(5): 588– https://doi.org/10.1038/s41401-019-0334-5 [PubMed] [PMC]
Kayser V, Christensen D (2000) Antinociceptive effect of systemic gabapentin in mononeuropathic rats, depends on stimulus characteristics and level of test integration. Pain 88(1): 53– https://doi.org/10.1016/S0304-3959(00)00307-9 [PubMed]
Kim SH, Kandiah N, Hsu JL, Suthisisang C, Udommongkol C, Dash A (2017) Beyond symptomatic effects: potential of donepezil as a neuroprotective agent and disease modifier in Alzheimer’s disease. British Journal of Pharmacology 174(23): 4224– https://doi.org/10.1111/bph.14030 [PubMed] [PMC]
Leer A, Haesen K, Vervliet B (2018) Beyond extinction: Prolonged conditioning and repeated threat exposure abolish contextual renewal of fear-potentiated startle discrimination but leave expectancy ratings intact. Frontiers in Psychiatry 9: 117. https://doi.org/10.3389/fpsyt.2018.00117 [PubMed] [PMC]
Liu Y, Ye S, Li XN, Li WG (2024) Memory trace for fear extinction: Fragile yet reinforceable. Neuroscience Bulletin 40(6): 777– https://doi.org/10.1007/s12264-023-01129-3 [PubMed] [PMC]
Lu B, Nagappan G, Lu Y (2014) BDNF and synaptic plasticity, cognitive function, and dysfunction. Handbook of Experimental Pharmacology 220: 223–25 https://doi.org/10.1007/978-3-642-45106-5_9 [PubMed]
Mahdi O, Chiroma SM, Hidayat Baharuldin MT, Mohd Nor NH, Mat Taib CN, Jagadeesan S, Devi S, Mohd Moklas MA (2021) WIN55,212-2 attenuates cognitive impairments in AlCl3 + d-galactose-induced Alzheimer’s disease rats by enhancing neurogenesis and reversing oxidative stress. Biomedicines 9(9): 1270. https://doi.org/10.3390/biomedicines9091270 [PubMed] [PMC]
Martin R, Kuzniecky R, Ho S, Hetherington H, Pan J, Sinclair K, Gilliam F, Faught E (1999) Cognitive effects of topiramate, gabapentin, and lamotrigine in healthy young adults. Neurology 52(2): 321–32 https://doi.org/10.1212/WNL.52.2.321 [PubMed]
Mei D, Wang F, Yuan B, Lai M, Zhou Y, Cui W, Liu H, Zhou W (2023) Cognitive anhancer donepezil attenuates heroin-seeking behavior induced by cues in rats. Journal of Integrative Neuroscience 22(3): 76.https://doi.org/10.31083/j.jin2203076 [PubMed]
Miranda M, Morici JF, Zanoni MB, Bekinschtein P (2019) Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Frontiers in Cellular Neuroscience 13: 363. https://doi.org/10.3389/fncel.2019.00363 [PubMed] [PMC]
Moriarty O, Lang Y, Idrees Z, McGuire BE, Finn DP (2016) Impaired cued and spatial learning performance and altered cannabinoid CB₁ receptor functionality in the substantia nigra in a rat model of diabetic neuropathy. Behavioural Brain Research 303: 61– https://doi.org/10.1016/j.bbr.2016.01.027 [PubMed]
Nahdi AMTA, John A, Raza H(2017) Elucidation of molecular mechanisms of streptozotocin-induced oxidative stress, Apoptosis, and Mitochondrial Dysfunction in Rin-5F Pancreatic β-Cells. Oxidative Medicine and Cellular Longevity https://doi.org/10.1155/2017/7054272 [PubMed] [PMC]
Naranjo C, Ortega-Jiménez P, Del Reguero L, Moratalla G, Failde I (2020) Relationship between diabetic neuropathic pain and comorbidity. Their impact on pain intensity, diabetes complications and quality of life in patients with type-2 diabetes mellitus. Diabetes Research and Clinical Practice 165: 108236. https://doi.org/10.1016/j.diabres.2020.108236 [PubMed]
Nirogi R, Goura V, Shanmuganathan D, Jayarajan P, Abraham R (2012) Comparison of manual and automated filaments for evaluation of neuropathic pain behavior in rats. Journal of Pharmacological and Toxicological Methods 66(1): 8– https://doi.org/10.1016/j.vascn.2012.04.006 [PubMed]
Nirogi R, Jabaris SL, Jayarajan P, Abraham R, Shanmuganathan D, Rasheed MA, Royapalley PK, Goura V (2011) Antinociceptive activity of α4β2* neuronal nicotinic receptor agonist A-366833 in experimental models of neuropathic and inflammatory pain. European Journal of Pharmacology 668(1-2): 155– https://doi.org/10.1016/j.ejphar.2011.06.032 [PubMed]
Novello BJ, Pobre T (2025) Electrodiagnostic evaluation of peripheral neuropathy. StatPearls, Island, StatPearls Publishing. [PMC]
Oh G, Moga DC, Fardo DW, Abner EL (2022) The association of gabapentin initiation and neurocognitive changes in older adults with normal cognition. Frontiers in Pharmacology 13: 910719. https://doi.org/10.3389/fphar.2022.910719 [PubMed] [PMC]
Ong WY, Stohler CS, Herr DR (2019) Role of the prefrontal cortex in pain processing. Molecular Neurobiology56(2): 1137–1166. https://doi.org/10.1007/s12035-018-1130-9 [PubMed] [PMC]
Palomo-Osuna J, Dueñas M, Naranjo C, De Sola H, Salazar A, Failde I (2022) Factors related to cognitive function in type-2 diabetes and neuropathic pain patients, the role of mood and sleep disorders in this relationship. Scientific Reports 12(1): 15442. https://doi.org/10.1038/s41598-022-18949-4 [PubMed] [PMC]
Park CM, Inouye SK, Marcantonio ER, Metzger E, Bateman BT, Lie JJ, Lee SB, Levin R, Kim DH (2022) Perioperative gabapentin use and in-hospital adverse clinical events among older adults after major surgery. JAMA Internal Medicine 182(11): 1117– https://doi.org/10.1001/jamainternmed.2022.3680 [PubMed] [PMC]
Perez-García GS, Meneses A (2005) Effects of the potential 5-HT7 receptor agonist AS 19 in an autoshaping learning task. Behavioural Brain Research 163(1): 136– https://doi.org/10.1016/j.bbr.2005.04.014 [PubMed]
Povedano M, Gascón J, Gálvez R, Ruiz M, Rejas J (2007) Cognitive function impairment in patients with neuropathic pain under standard conditions of care. Journal of Pain and Symptom Management 33(1): 78– https://doi.org/10.1016/j.jpainsymman.2006.07.012 [PubMed]
Puig MV, Gulledge AT (2011) Serotonin and prefrontal cortex function: neurons, networks, and circuits. Molecular Neurobiology 44(3): 449–4 https://doi.org/10.1007/s12035-011-8214-0 [PubMed] [PMC]
Qian X, Yue L, Mellor D, Robbins NM, Li W, Xiao S (2022) Reduced peripheral nerve conduction velocity is associated with Alzheimer’s disease: A cross-sectional study from China. Neuropsychiatric Disease and Treatment 18: 231– https://doi.org/10.2147/NDT.S349005 [PubMed] [PMC]
Roberts FL, Cataldo LR, Fex M (2023) Monoamines’ role in islet cell function and type 2 diabetes risk. Trends in Molecular Medicine 29(12): 1045– https://doi.org/10.1016/j.molmed.2023.08.009 [PubMed]
Salinsky MC, Binder LM, Oken BS, Storzbach D, Aron CR, Dodrill CB (2002) Effects of gabapentin and carbamazepine on the EEG and cognition in healthy volunteers. Epilepsia 43(5): 482– https://doi.org/10.1046/j.1528-1157.2002.22501.x [PubMed]
Santello M, Bisco A, Nevian NE, Lacivita E, Leopoldo M, Nevian T (2017) The brain-penetrant 5-HT7 receptor agonist LP-211 reduces the sensory and affective components of neuropathic pain. Neurobiology of Disease 106: 214– https://doi.org/10.1016/j.nbd.2017.07.005 [PubMed] [PMC]
Schreiber AK, Nones CF, Reis RC, Chichorro JG, Cunha JM (2015) Diabetic neuropathic pain: Physiopathology and treatment. World Journal of Diabetes 6(3): 432– https://doi.org/10.4239/wjd.v6.i3.432 [PubMed] [PMC]
Shillo P, Sloan G, Greig M, Hunt L, Selvarajah D, Elliott J, Gandhi R, Wilkinson ID, Tesfaye S (2019) Painful and painless diabetic neuropathies: What is the difference? Current Diabetes Reports 19 (6): 32. https://doi.org/10.1007/s11892-019-1150-5 [PubMed] [PMC]
Solís-Guillén R, Leopoldo M, Meneses A, Centurión D (2021) Activation of 5-HT1A and 5-HT7 receptors enhanced a positively reinforced long-term memory. Behavioural Brain Research 397: 112932. https://doi.org/10.1016/j.bbr.2020.112932 [PubMed]
Takemiya T, Fumizawa K, Yamagata K, Iwakura Y, Kawakami M (2017) Brain interleukin-1 facilitates learning of a water maze spatial memory task in young mice. Frontiers in Behavioral Neuroscience 11: 202. https://doi.org/10.3389/fnbeh.2017.00202 [PubMed] [PMC]
Tesfaye S, Boulton AJ, Dickenson AH (2013) Mechanisms and management of diabetic painful distal symmetrical polyneuropathy. Diabetes Care 36(9): 2456– https://doi.org/10.2337/dc12-1964 [PubMed] [PMC]
Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P (2010). Toronto diabetic neuropathy expert group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 33(10): 2285– https://doi.org/10.2337/dc10-1303 [PubMed] [PMC]
Tesfaye S, Kempler P (2023) Conventional management and current guidelines for painful diabetic neuropathy.Diabetes Research and Clinical Practice Suppl 1: 110765. https://doi.org/10.1016/j.diabres.2023.110765 [PubMed]
Tong L, Prieto GA, Kramár EA, Smith ED, Cribbs DH, Lynch G, Cotman CW (2012) Brain-derived neurotrophic factor-dependent synaptic plasticity is suppressed by interleukin-1β via p38 mitogen-activated protein kinase. Journal of Neuroscience 32 (49): 17714–177 https://doi.org/10.1523/jneurosci.1253-12.2012 [PubMed] [PMC]
Upadhyay A, Boyle KE, Broderick TL (2021) The effects of streptozotocin-induced diabetes and insulin treatment on carnitine biosynthesis and renal excretion. Molecules 26 (22): 6872. https://doi.org/10.3390/molecules26226872 [PubMed] [PMC]
Villegas AQ, Manzo HSA, Oa Valenzuela Almada M, Modragon CB, Guzman RG (2019) Procognitive and neuroprotective effect of 5-HT7 agonist in an animal model by ICV amyloid-b injection (P1.1-005). Neurology Issue 92 (suppl 15) https://doi.org/10.1212/WNL.92.15_supplement.P1.1-005
Wiffen PJ, Derry S, Bell RF, Rice AS, Tölle TR, Phillips T, Moore RA (2017) Gabapentin for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews 6(6): CD007938. https://doi.org/10.1002/14651858.cd007938.pub4 [PubMed] [PMC]
Yoon C, Wook YY, Sik NH, Ho KS, Mo CJ (1994) Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 59(3): 369– https://doi.org/10.1016/0304-3959(94)90023-x [PubMed]
Zheng H, Lin Q, Wang D, Xu P, Zhao L, Hu W, Bai G, Yan Z, Gao H (2017) NMR-based metabolomics reveals brain region-specific metabolic alterations in streptozotocin-induced diabetic rats with cognitive dysfunction. Metabolic Brain Disease 32(2): 585– https://doi.org/10.1007/s11011-016-9949-0 [PubMed]
Zhu X, Zhang C, Hu Y, Wang Y, Xiao S, Zhu Y, Sun H, Sun J, Xu C, Xu Y, Chen Y, He X, Liu B, Liu J, Du J, Liang Y, Liu B, Li X, Jiang Y, Shen Z, Shao X, Fang J (2024) Modulation of comorbid chronic neuropathic pain and anxiety-like behaviors by glutamatergic neurons in the ventrolateral periaqueductal gray and the analgesic and anxiolytic effects of electroacupuncture. eNeuro 11(8): ENEURO.0454-23.2024. https://doi.org/10.1523/eneuro.0454-23.2024 [PubMed] [PMC]
Additional Files
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Goura V, Jayarajan P, Abraham R, Medapati R, Kallepalli R, Kishore A, Nirogi R

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

