FIMD based model validation approaches for Rheumatoid arthritis and associated cardiovascular complications: an attempt towards translational competence
DOI:
https://doi.org/10.18413/rrpharmacology.10.446Аннотация
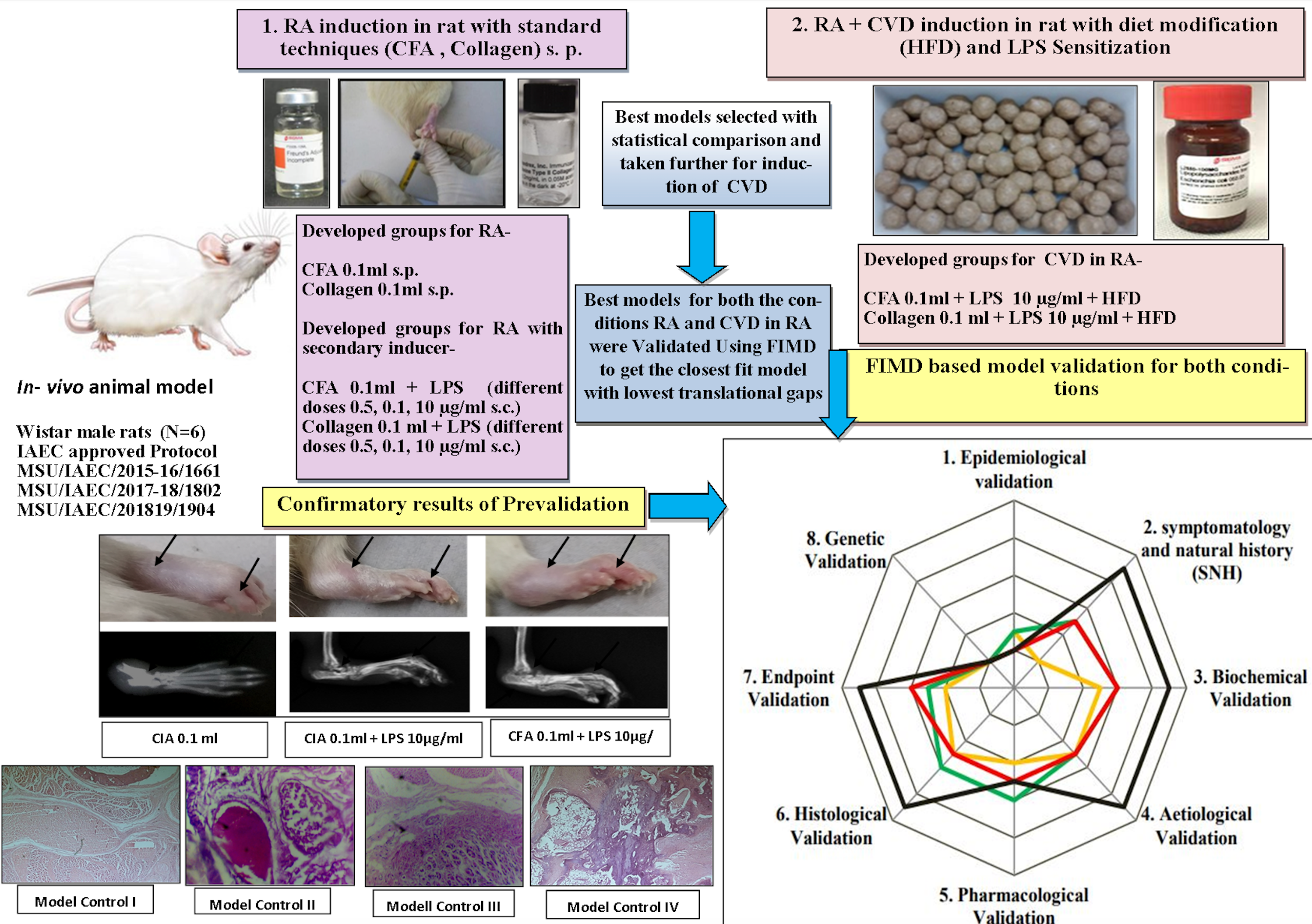
Introduction: The present in-vivo experiment was designed to validate newly developed rat models for Rheumatoid Arthritis (RA) as well as cardiovascular complications in RA for translational competence of disease with clinical situation. The FIMD (Framework to Identify Models for Disease) method based on etiology, pathophysiology, symptomatology, and response to therapeutic interventions was used to compare these models sensitized with primary (CFA, bovine collagen type II) and secondary inducing agents (high fat diet, lipopolysaccharide) in different combinations.
Materials and Methods: Twenty-four Wistar male rats (four groups/n=6) were taken after prevalidation, where a large dataset was analyzed by using statistical methods (one way ANOVA, repeated Measure ANOVA). Among these groups, two best models from RA representative groups and two best models from cardiovascular complications in RA generated groups were taken for final comparison and validation by the same weight score method for obtaining a percentage similarity and validity score using a radar plot based on FIMD to get the best suited model.
Results and Discussion: The findings of this study on the basis of FIMD showed that the collagen (0.1 mL)+lipopolysaccharide (10 µg/mL) induced model is closely fit for preclinical events of RA, with the highest validity score (82%) among all groups and the collagen (0.1 mL)+ lipopolysaccharide (10 µg/mL)+HFD represents a validated model (95%) for co-morbid cardiovascular complications in RA.
Conclusion: The developed and validated models were a fresh attempt using different inducers sequentially to culminate the emerging issue of extra organ manifestation in existing RA via a similar pathway, which can be a contributor to future research in drug discoveries in pharmacology.
Графическая аннотация
Ключевые слова:
adjuvant (CFA), collagen, high fat diet, lipopolysaccharid, rheumatoid arthritis, validationБиблиографические ссылки
Barnett MJ, Doroudgar S, Khosraviani VIp EJ (2022) Multiple comparisons: To compare or not to compare, that is the question. Research in Social and Administrative Pharmacy 18(2): 2331–2334. https://doi.org/10.1016/j.sapharm.2021.07.006 [PubMed]
Cassotta M, Pistollato F, Battino M (2020) Rheumatoid arthritis research in the 21stcentury: Limitations of traditional models, new technologies, and opportunities for a human biology-based approach. ALTEX-Alternatives to Animal Experimentation 37(2): 223–242. https://doi.org/10.14573/altex.1910011 [PubMed]
Deore AB, Dhumane JR, Wagh R, Sonawane R (2019) The stages of drug discovery and development process. Asian Journal of Pharmaceutical Research and Development 7(6): 62–67. https://doi.org/10.1111/bph.15178
Dobbin KK, Cesano A, Alvarez J, Hawtin R, Janetzki S, Kirsch I, Masucci GV, Robbins PB, Selvan SR, Streicher HZ (2016) Validation of biomarkers to predict response to immunotherapy in cancer: Volume II – clinical validation and regulatory considerations. Journal for Immunotherapy of Cancer 4(1): 1–14. https://doi.org/10.1186/s40425-016-0178-1 [PubMed] [PMC]
Dubey T, Chorawala M (2015) Lipopolysaccharide (LPS) and Muramyl dipeptide (MDP) induced chronic liver injury in high fat diet fed rat-a possible model for non alcoholic fatty liver disease. International Journal of Pharmaceutical Sciences and Research 6(8): 3401. https://doi.org/10.13040/IJPSR.0975-8232.6(8).3401-11
Ferreira GS, Veening-Griffioen DH, Boon WP, Moors EH, Gispen-de Wied CC, Schellekens H, van Meer PJ (2019) Correction: A standardized framework to identify optimal animal models for efficacy assessment in drug development. PloS One 14(7): e0220325. https://doi.org/10.1371/journal.pone.0218014 [PubMed] [PMC]
Ferreira GS, Veening-Griffioen DH, Boon WP, Moors EH, van Meer PJ (2020) Levelling the translational gap for animal to human efficacy data. Animals 10(7): 1199. https://doi.org/doi:10.3390/ani10071199 [PubMed] [PMC]
Hong J-I, Park IYKim HA (2020) Understanding the molecular mechanisms underlying the pathogenesis of arthritis pain using animal models. International Journal of Molecular Sciences 21(2): 533. https://doi.org/10.3390/ijms21020533 [PubMed] [PMC]
Kollias G, Papadaki P, Apparailly F, Vervoordeldonk MJ, Holmdahl R, Baumans V, Desaintes C, Di Santo J, Distler J, Garside P (2011) Animal models for arthritis: Innovative tools for prevention and treatment. Annals of the Rheumatic Diseases 70(8): 1357–1362. http://dx.doi.org/10.1136/ard.2010.148551 [PubMed]
Lilley E, Stanford SC, Kendall DE, Alexander SP, Cirino G, Docherty JR, George CH, Insel PA, Izzo AA, Ji Y (2020) Arrive 2.0 and the British journal of pharmacology: Updated guidance for 2020. British Journal of Pharmacology 177(16): 3611. https://doi.org/10.1111/bph.15178 [PubMed] [PMC]
Luo X, Cui J, Long XChen Z (2020) TLRs play crucial roles in regulating RA synoviocyte. Endocrine, Metabolic & Immune Disorders-Drug Targets 20(8): 1156–1165. https://doi.org/10.2174/1871530320666200427115225 [PubMed]
Miyoshi M, Liu S (2024) Collagen-induced Arthritis Models Rheumatoid Arthritis: Methods and Protocols. Springer, pp. 3–7. https://doi.org/10.1007/978-1-4939-8802-0_1
Sams-Dodd F (2006) Strategies to optimize the validity of disease models in the drug discovery process. Drug Discovery Today 11(7-8): 355–363. https://doi.org/10.1016/j.drudis.2006.02.005 [PubMed]
Scannell JW, Bosley J, Hickman JA, Dawson GR, Truebel H, Ferreira GS, Richards D, Treherne JM (2022) Predictive validity in drug discovery: What it is, why it matters and how to improve it. Nature Reviews Drug Discovery 21(12): 915–931. https://doi.org/10.1038/s41573-022-00552-x [PubMed]
Silverman J, Nithianantharajah J, Der-Avakian A, Young J, Rizzo SS (2020) Lost in translation: At the crossroads of face validity and translational utility of behavioral assays in animal models for the development of therapeutics. Neuroscience and Biobehavioral Reviews 116: 452. https://doi.org/10.1016/j.neubiorev.2020.07.008 [PubMed] [PMC]
Tadenev AL, Burgess RW (2019) Model validity for preclinical studies in precision medicine: Precisely how precise do we need to be? Mammalian Genome 30(5): 111–122. https://doi.org/10.1007/s00335-019-09798-0 [PubMed] [PMC]
Загрузки
Дополнительные файлы
Опубликован
Как цитировать
Выпуск
Раздел
Лицензия
Copyright (c) 2024 Trupti Dubey, Kirti V. Patel

Это произведение доступно по лицензии Creative Commons «Attribution» («Атрибуция») 4.0 Всемирная.
 Русский
Русский
 English
English

