Molecular mechanisms of wound healing: the role of zinc as an essential microelement
DOI:
https://doi.org/10.18413/rrpharmacology.9.10003Abstract
Introduction: In the course of evolution, humans developed a number of complex multi-step wound healing mechanisms which limit the infectious agents access to the bloodstream, protect the organism from blood loss, and restore skin integrity. The process of skin wound healing includes the following stages: haemostasis, inflammation, proliferation, and remodeling. These processes are possible because of modulators, growth factors, cytokines, matrix metalloproteinases and cellular receptors, as well as some trace elements like zinc.
Materials and Methods: The presented data was analyzed and compiled using all relevant articles describing the role of zinc in blood coagulation, proliferation, damaged tissues regeneration and angiogenesis.
Results and Discussion: There are some on-going studies about zinc effects on blood coagulation, proliferation, damaged tissues regeneration and angiogenesis. However, molecular mechanisms of these processes are not yet fully understood and require further study. The analysis of scientific efforts to investigate the role of zinc in wound healing molecular mechanisms is especially relevant to the understanding of treatment of skin wound injuries.
Conclusion: Wound healing is a complex multi-phase process consisting of several phases. Each stage involves metal ions, primarily zinc, which stimulates re-epithelialization, decreases inflammation and bacterial growth. The use of known zinc-based drugs is accompanied by side effects and low efficacy due to low skin absorption. These factors significantly limit use of such drugs and highlight the urgency of finding new, more effective and safe treatment. The emerging field of nanobiotechnology may provide an alternative platform to develop new therapeutic agents for the wound healing process.
Graphical Abstract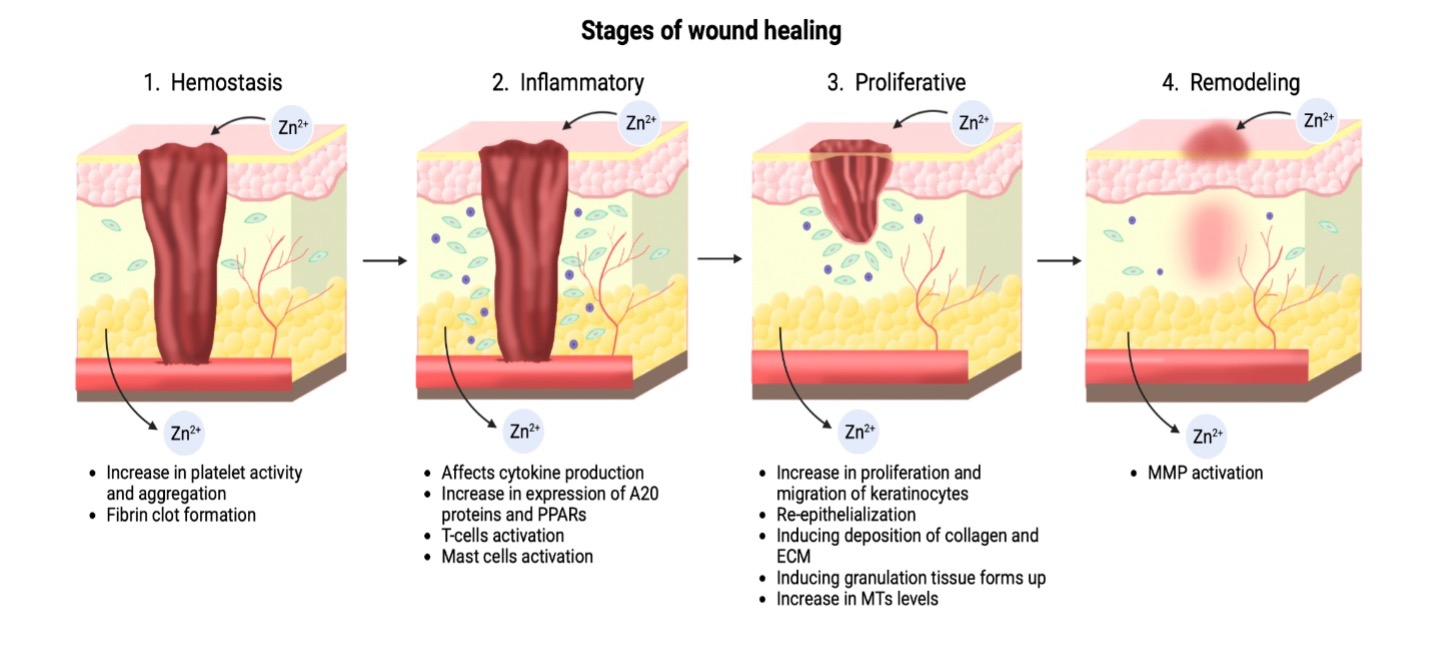
Graphical Abstract

Keywords:
haemostasis, inflammation, proliferation, remodeling, skin, wound healing, zincReferences
Abdullah BJ, Atasoy N, Omer AK (2019) Evaluate the effects of platelet rich plasma (PRP) and zinc oxide ointment on skin wound healing. Annals of Medicine and Surgery (2012) 37: 30–37. https://doi.org/10.1016/j.amsu.2018.11.009[PubMed] [PMC]
Adjepong M, Agbenorku P, Brown P, Oduro I (2016) The role of antioxidant micronutrients in the rate of recovery of burn patients: a systematic review. Burns & Trauma 4: 18. https://doi.org/10.1186/s41038-016-0044-x [PubMed] [PMC]
Agren MS (1993) Zinc oxide increases degradation of collagen in necrotic wound tissue. British Journal of Dermatology 129(2): 221–222. https://doi.org/10.1111/j.1365-2133.1993.tb03533.x [PubMed]
Agren MS, Auf dem Keller U (2020) Matrix metalloproteinases: How much can they do? International Journal of Molecular Sciences 21(8): 2678. https://doi.org/10.3390/ijms21082678 [PubMed] [PMC]
Ahmed NS, Lopes-Pires M, Pugh N (2021) Zinc: an endogenous and exogenous regulator of platelet function during hemostasis and thrombosis. Platelets 32(7): 880–887. https://doi.org/10.1080/09537104.2020.1840540 [PubMed]
Ahmed NS, Lopes Pires ME, Taylor KA, Pugh N (2019) Agonist-evoked increases in intra-platelet zinc couple to functional responses. Thrombosis and Haemostasis 119(1): 128–139. https://doi.org/10.1055/s-0038-1676589 [PubMed] [PMC]
Ahmed R, Tariq M, Ali I, Asghar R, Noorunnisa Khanam P, Augustine R, Hasan A (2018) Novel electrospun chitosan/polyvinyl alcohol/zinc oxide nanofibrous mats with antibacterial and antioxidant properties for diabetic wound healing. International Journal of Biological Macromolecules 120(Pt A): 385–393. https://doi.org/10.1016/j.ijbiomac.2018.08.057 [PubMed]
Apgar J (1968) Effect of zinc deficiency on parturition in the rat. The American Journal of Physiology 215(1): 160–163. https://doi.org/10.1152/ajplegacy.1968.215.1.160 [PubMed]
Augustine R, Dominic EA, Reju I, Kaimal B, Kalarikkal N, Thomas S (2014) Investigation of angiogenesis and its mechanism using zinc oxide nanoparticle-loaded electrospun tissue engineering scaffolds. RSC Advances 4: 51528–51536. https://doi.org/10.1039/C4RA07361D
Baghaie S, Khorasani MT, Zarrabi A, Moshtaghian J (2017) Wound healing properties of PVA/starch/chitosan hydrogel membranes with nano Zinc oxide as antibacterial wound dressing material. Journal of Biomaterials Science, Polymer Edition 28(18): 2220–2241. https://doi.org/10.1080/09205063.2017.1390383 [PubMed]
Bin B-H, Seo J, Kim ST (2018) Function, structure, and transport aspects of ZIP and ZnT zinc transporters in immune cells. Journal of Immunology Research 2018: 9365747. https://doi.org/10.1155/2018/9365747 [PubMed] [PMC]
Bin B-H, Bhin J, Kim N-H, Lee S-H, Jung H-S, Seo J, Kim D-K, Hwang D, Fukada T, Lee A-Y, Lee TR, Cho E-G (2017) An acrodermatitis enteropathica-associated Zn transporter, ZIP4, regulates human epidermal homeostasis. The Journal of Investigative Dermatology 137(4): 874–883. https://doi.org/10.1016/j.jid.2016.11.028 [PubMed]
Bretón-Romero R, Lamas S (2014) Hydrogen peroxide signaling in vascular endothelial cells. Redox Biology 2: 529–534. https://doi.org/10.1016/j.redox.2014.02.005 [PubMed] [PMC]
von Bülow V, Dubben S, Engelhardt G, Hebel S, Plümäkers B, Heine H, Rink L, Haase H (2007) Zinc-dependent suppression of TNF-alpha production is mediated by protein kinase A-induced inhibition of Raf-1, I kappa B kinase beta, and NF-kappa B. Journal of Immunology 179(6): 4180–4186. https://doi.org/10.4049/jimmunol.179.6.4180 [PubMed]
Chaudhry SA, Serrata M, Tomczak L, Higgins S, Ryu J, Laprise D, Enjyoji K, Bekendam R, Kaushik V, Flaumenhaft R, Bendapudi PK (2020) Cationic zinc is required for factor XII recruitment and activation by stimulated platelets and for thrombus formation in vivo. Journal of Thrombosis and Haemostasis 18(9): 2318–2328. https://doi.org/10.1111/jth.14964[PubMed] [PMC]
Cho JG, Park S, Lim CH, Kim HS, Song SY, Roh T-Y, Sung J-H, Suh W, Ham S-J, Lim K-H, Park SG (2016) ZNF224, Krüppel like zinc finger protein, induces cell growth and apoptosis-resistance by down-regulation of p21 and p53 via miR-663a. Oncotarget 7(21): 31177–31190. https://doi.org/10.18632/oncotarget.8870 [PubMed] [PMC]
Cleetus CM, Alvarez Primo F, Fregoso G, Lalitha Raveendran N, Noveron JC, Spencer CT, Ramana CV, Joddar B (2020) Alginate hydrogels with embedded ZnO nanoparticles for wound healing therapy. International Journal of Nanomedicine 15: 5097–5111. https://doi.org/10.2147/IJN.S255937 [PubMed] [PMC]
Coger V, Million N, Rehbock C, Sures B, Nachev M, Barcikowski S, Wistuba N, Strauß S, Vogt PM (2019) Tissue concentrations of zinc, iron, copper, and magnesium during the phases of full thickness wound healing in a rodent model. Biological Trace Element Research 191(1): 167–176. https://doi.org/10.1007/s12011-018-1600-y [PubMed] [PMC]
Cui N, Hu M, Khalil RA (2017) Biochemical and biological attributes of matrix metalloproteinases. Progress in Molecular Biology and Translational Science 147: 1–73. https://doi.org/10.1016/bs.pmbts.2017.02.005 [PubMed] [PMC]
Diegelmann RF, Evans MC (2004) Wound healing: an overview of acute, fibrotic and delayed healing. Frontiers in Bioscience: A Journal and Virtual Library 9: 283–289. https://doi.org/10.2741/1184 [PubMed]
Díez-Pascual AM, Díez-Vicente AL (2015) Wound healing bionanocomposites based on castor oil polymeric films reinforced with chitosan-modified ZnO nanoparticles. Biomacromolecules 16(9): 2631–2644. https://doi.org/10.1021/acs.biomac.5b00447 [PubMed]
Dudev T, Lim C (2003) Principles governing Mg, Ca, and Zn binding and selectivity in proteins. Chemical Reviews 103(3): 773–788. https://doi.org/10.1021/cr020467n [PubMed]
Emery MP, Browning JD, O’Dell BL (1990) Impaired hemostasis and platelet function in rats fed low zinc diets based on egg white protein. The Journal of Nutrition 120(9): 1062–1067. https://doi.org/10.1093/jn/120.9.1062 [PubMed]
Feng X, Zhou S, Cai W, Guo J (2021) The miR-93-3p/ZFP36L1/ZFX axis regulates keratinocyte proliferation and migration during skin wound healing. Molecular Therapy. Nucleic Acids 23: 450–463. https://doi.org/10.1016/j.omtn.2020.11.017 [PubMed] [PMC]
Fukada T, Kambe T (2011) Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics: Integrated Biometal Science 3(7): 662–674. https://doi.org/10.1039/c1mt00011j [PubMed]
Gladka MM, Kohela A, Molenaar B, Versteeg D, Kooijman L, Monshouwer-Kloots J, Kremer V, Vos HR, Huibers MMH, Haigh JJ, Huylebroeck D, Boon RA, Giacca M, van Rooij E (2021) Cardiomyocytes stimulate angiogenesis after ischemic injury in a ZEB2-dependent manner. Nature Communications 12(1): 84. https://doi.org/10.1038/s41467-020-20361-3 [PubMed] [PMC]
Gupta M, Mahajan VK, Mehta KS, Chauhan PS (2014) Zinc therapy in dermatology: a review. Dermatology Research and Practice 2014: 709152. https://doi.org/10.1155/2014/709152 [PubMed] [PMC]
Gupta M, Mahajan VK, Mehta KS, Chauhan PS, Rawat R (2015) Peroxisome proliferator-activated receptors (PPARs) and PPAR agonists: the “future” in dermatology therapeutics? Archives of Dermatological Research 307(9): 767–780. https://doi.org/10.1007/s00403-015-1571-1 [PubMed]
Haase H, Ober-Blöbaum JL, Engelhardt G, Hebel S, Heit A, Heine H, Rink L (2008) Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. Journal of Immunology 181(9): 6491–6502. https://doi.org/10.4049/jimmunol.181.9.6491 [PubMed]
Hara T, Takeda T-A, Takagishi T, Fukue K, Kambe T, Fukada T (2017) Physiological roles of zinc transporters: molecular and genetic importance in zinc homeostasis. The Journal of Physiological Sciences 67(2): 283–301. https://doi.org/10.1007/s12576-017-0521-4 [PubMed]
Hayden DM, Forsyth C, Keshavarzian A (2011) The role of matrix metalloproteinases in intestinal epithelial wound healing during normal and inflammatory states. The Journal of Surgical Research 168(2): 315–324. https://doi.org/10.1016/j.jss.2010.03.002 [PubMed]
Hershfinkel M (2018) The zinc sensing receptor, ZnR/GPR39, in health and disease. International Journal of Molecular Sciences 19(2): 439. https://doi.org/10.3390/ijms19020439 [PubMed] [PMC]
Hoch E, Levy M, Hershfinkel M, Sekler I (2020) Elucidating the H+ coupled Zn2+ transport mechanism of ZIP4; Implications in acrodermatitis enteropathica. International Journal of Molecular Sciences 21(3): 734. https://doi.org/10.3390/ijms21030734 [PubMed] [PMC]
Hojyo S, Fukada T (2016) Zinc transporters and signaling in physiology and pathogenesis. Archives of Biochemistry and Biophysics 611: 43–50. https://doi.org/10.1016/j.abb.2016.06.020 [PubMed]
Hopmeier P, Halbmayer M, Fischer M, Marx G (1990) Zinc modulates thrombin adsorption to fibrin. Thrombosis Research 58(3): 293–301. https://doi.org/10.1016/0049-3848(90)90099-x [PubMed]
Jabłońska-Trypuć A, Matejczyk M, Rosochacki S (2016) Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. Journal of Enzyme Inhibition and Medicinal Chemistry 31: 177–183. https://doi.org/10.3109/14756366.2016.1161620 [PubMed]
Jin L, Murakami TH, Janjua NA, Hori Y (1994) The effects of zinc oxide and diethyldithiocarbamate on the mitotic index of epidermal basal cells of mouse skin. Acta Medica Okayama 48(5): 231–236. https://doi.org/10.18926/AMO/31117 [PubMed]
Kambe T (2012) Molecular architecture and function of ZnT transporters. Current Topics in Membranes 69: 199–220. https://doi.org/10.1016/B978-0-12-394390-3.00008-2 [PubMed]
Kambe T, Matsunaga M, Takeda T (2017) Understanding the contribution of zinc transporters in the function of the early secretory pathway. International Journal of Molecular Sciences 18(10): 2179. https://doi.org/10.3390/ijms18102179[PubMed] [PMC]
Kang SU, Choi JW, Chang JW, Kim KI, Kim YS, Park JK, Kim YE, Lee YS, Yang SS, Kim C-H (2017) N2 non-thermal atmospheric pressure plasma promotes wound healing in vitro and in vivo: Potential modulation of adhesion molecules and matrix metalloproteinase-9. Experimental Dermatology 26(2): 163–170. https://doi.org/10.1111/exd.13229[PubMed]
Kantipudi S, Sunkara JR, Rallabhandi M, Thonangi CV, Cholla RD, Kollu P, Parvathaneni MK, Pammi SVN (2018) Enhanced wound healing activity of Ag-ZnO composite NPs in Wistar Albino rats. IET Nanobiotechnology 12(4): 473–478. https://doi.org/10.1049/iet-nbt.2017.0087 [PubMed] [PMC]
Kaushik M, Niranjan R, Thangam R, Madhan B, Pandiyarasan V, Ramachandran C, Oh D-H, Venkatasubbu GD (2019) Investigations on the antimicrobial activity and wound healing potential of ZnO nanoparticles. Applied Surface Science 479: 1169–1177. https://doi.org/10.1016/j.apsusc.2019.02.189 [PubMed]
Kimura T, Kambe T (2016) The functions of metallothionein and ZIP and ZnT transporters: An overview and perspective. International Journal of Molecular Sciences 17(3): 336. https://doi.org/10.3390/ijms17030336 [PubMed] [PMC]
Kiran Gotru S, van Geffen JP, Nagy M, Mammadova-Bach E, Eilenberger J, Volz J, Manukjan G, Schulze H, Wagner L, Eber S, Schambeck C, Deppermann C, Brouns S, Nurden P, Greinacher A, Sachs U, Nieswandt B, Hermanns HM, Heemskerk JWM, Braun A (2019) Defective Zn2+ homeostasis in mouse and human platelets with α- and δ-storage pool diseases. Scientific Reports 9: 8333. https://doi.org/10.1038/s41598-019-44751-w [PubMed] [PMC]
Klein C, Heyduk T, Sunahara RK (2004) Zinc inhibition of adenylyl cyclase correlates with conformational changes in the enzyme. Cellular Signalling 16(10): 1177–1185. https://doi.org/10.1016/j.cellsig.2004.03.008 [PubMed]
Kogan S, Sood A, Garnick MS (2017) Zinc and wound healing: A review of zinc physiology and clinical applications. Wounds 29(4): 102–106. [PubMed]
Komi DEA, Khomtchouk K, Santa Maria PL (2020) A review of the contribution of mast cells in wound healing: involved molecular and cellular mechanisms. Clinical Reviews in Allergy & Immunology 58(3): 298–312. https://doi.org/10.1007/s12016-019-08729-w [PubMed]
Korbecki J, Bobiński R, Dutka M (2019) Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflammation Research 68(6): 443–458. https://doi.org/10.1007/s00011-019-01231-1 [PubMed] [PMC]
Krarup P-M, Eld M, Jorgensen LN, Hansen MB, Agren MS (2017) Selective matrix metalloproteinase inhibition increases breaking strength and reduces anastomotic leakage in experimentally obstructed colon. International Journal of Colorectal Disease 32(9): 1277–1284. https://doi.org/10.1007/s00384-017-2857-x [PubMed]
Krishnaswamy VR, Mintz D, Sagi I (2017) Matrix metalloproteinases: The sculptors of chronic cutaneous wounds. Biochimica Et Biophysica Acta. Molecular Cell Research 1864: 2220–2227. https://doi.org/10.1016/j.bbamcr.2017.08.003 [PubMed]
Kurmis R, Greenwood J, Aromataris E (2016) Trace element supplementation following severe burn injury: A systematic review and meta-analysis. Journal of Burn Care & Research: Official Publication of the American Burn Association 37(3): 143–159. https://doi.org/10.1097/BCR.0000000000000259 [PubMed]
Kuźmicka W, Manda-Handzlik A, Cieloch A, Mroczek A, Demkow U, Wachowska M, Ciepiela O (2020) Zinc supplementation modulates nets release and neutrophils’ degranulation. Nutrients 13(1): 51. https://doi.org/10.3390/nu13010051 [PubMed] [PMC]
Kyriakides TR, Wulsin D, Skokos EA, Fleckman P, Pirrone A, Shipley JM, Senior RM, Bornstein P (2009) Mice that lack matrix metalloproteinase-9 display delayed wound healing associated with delayed reepithelization and disordered collagen fibrillogenesis. Matrix Biology: Journal of the International Society for Matrix Biology 28(2): 65–73. https://doi.org/10.1016/j.matbio.2009.01.001 [PubMed] [PMC]
Lamore SD, Cabello CM, Wondrak GT (2010) The topical antimicrobial zinc pyrithione is a heat shock response inducer that causes DNA damage and PARP-dependent energy crisis in human skin cells. Cell Stress & Chaperones 15(3): 309–322. https://doi.org/10.1007/s12192-009-0145-6 [PubMed] [PMC]
Larsen HF, Ahlström MG, Gjerdrum LMR, Mogensen M, Ghathian K, Calum H, Sørensen AL, Forman JL, Vandeven M, Holerca MN, Du-Thumm L, Jorgensen LN, Agren MS (2017) Noninvasive measurement of reepithelialization and microvascularity of suction-blister wounds with benchmarking to histology. Wound Repair and Regeneration 25(6): 984–993. https://doi.org/10.1111/wrr.12605 [PubMed]
Lee S, Eskin SG, Shah AK, Schildmeyer LA, McIntire LV (2012) Effect of zinc and nitric oxide on monocyte adhesion to endothelial cells under shear stress. Annals of Biomedical Engineering 40(3): 697–706. https://doi.org/10.1007/s10439-011-0434-y [PubMed] [PMC]
Lin P-H, Sermersheim M, Li H, Lee PHU, Steinberg SM, Ma J (2017) Zinc in wound healing modulation. Nutrients 10(1): 16. https://doi.org/10.3390/nu10010016 [PubMed] [PMC]
Liu T, Zhang L, Joo D, Sun S-C (2017) NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy 2: 17023. https://doi.org/10.1038/sigtrans.2017.23 [PubMed] [PMC]
Lopes-Pires ME, Ahmed NS, Vara D, Gibbins JM, Pula G, Pugh N (2021) Zinc regulates reactive oxygen species generation in platelets. Platelets 32(3): 368–377. https://doi.org/10.1080/09537104.2020.1742311 [PubMed]
Mammadova-Bach E, Braun A (2019) Zinc homeostasis in platelet-related diseases. International Journal of Molecular Sciences 20(21): 5258. https://doi.org/10.3390/ijms20215258 [PubMed] [PMC]
Manuja A, Raguvaran R, Kumar B, Kalia A, Tripathi BN (2020) Accelerated healing of full thickness excised skin wound in rabbits using single application of alginate/acacia based nanocomposites of ZnO nanoparticles. International Journal of Biological Macromolecules 155: 823–833. https://doi.org/10.1016/j.ijbiomac.2020.03.221 [PubMed]
Maret W (2013) Zinc biochemistry: from a single zinc enzyme to a key element of life. Advances in Nutrition 4(1): 82–91. https://doi.org/10.3945/an.112.003038 [PubMed] [PMC]
Marx G, Hopmeier P (1986) Zinc inhibits FPA release and increases fibrin turbidity. American Journal of Hematology 22(4): 347–353. https://doi.org/10.1002/ajh.2830220403 [PubMed]
Maxfield L, Shukla S, Crane JS (2022) Zinc Eficiency. In: StatPearls. StatPearls Publishing, Treasure Island (FL). Available at: http://www.ncbi.nlm.nih.gov/books/NBK493231/ (January 5, 2023)
Maywald M, Rink L (2017) Zinc supplementation induces CD4+CD25+Foxp3+ antigen-specific regulatory T cells and suppresses IFN-γ production by upregulation of Foxp3 and KLF-10 and downregulation of IRF-1. European Journal of Nutrition 56(5): 1859–1869. https://doi.org/10.1007/s00394-016-1228-7 [PubMed]
Maywald M, Meurer SK, Weiskirchen R, Rink L (2017) Zinc supplementation augments TGF-β1-dependent regulatory T cell induction. Molecular Nutrition & Food Research 61(3). https://doi.org/10.1002/mnfr.201600493 [PubMed]
Mendes C, Thirupathi A, Corrêa MEAB, Gu Y, Silveira PCL (2022) The use of metallic nanoparticles in wound healing: New perspectives. International Journal of Molecular Sciences 23(23): 15376. https://doi.org/10.3390/ijms232315376 [PubMed] [PMC]
Mirastschijski U, Haaksma CJ, Tomasek JJ, Agren MS (2004) Matrix metalloproteinase inhibitor GM 6001 attenuates keratinocyte migration, contraction and myofibroblast formation in skin wounds. Experimental Cell Research 299(2): 465–475. https://doi.org/10.1016/j.yexcr.2004.06.007 [PubMed]
Mirastschijski U, Martin A, Jorgensen LN, Sampson B, Agren MS (2013) Zinc, copper, and selenium tissue levels and their relation to subcutaneous abscess, minor surgery, and wound healing in humans. Biological Trace Element Research 153(1-3): 76–83. https://doi.org/10.1007/s12011-013-9658-z [PubMed]
Nakano Y, Arima T, Tobita Y, Uchiyama M, Shimizu A, Takahashi H (2020) Combination of peroxisome proliferator-activated receptor (PPAR) alpha and gamma agonists prevents corneal inflammation and neovascularization in a rat alkali burn model. International Journal of Molecular Sciences 21(14): 5093. https://doi.org/10.3390/ijms21145093 [PubMed] [PMC]
Nishida K, Uchida R (2017) [Regulatory Mechanism of Mast Cell Activation by Zinc Signaling]. Yakugaku Zasshi 137(5): 495–501. https://doi.org/10.1248/yakushi.16-00239-1 [PubMed] [in Japanese]
Nishida K, Uchida R (2018) Role of zinc signaling in the regulation of mast cell-, basophil-, and T cell-mediated allergic responses. Journal of Immunology Research 2018: 5749120. https://doi.org/10.1155/2018/5749120 [PubMed] [PMC]
Nishida K, Hasegawa A, Yamasaki S, Uchida R, Ohashi W, Kurashima Y, Kunisawa J, Kimura S, Iwanaga T, Watarai H, Hase K, Ogura H, Nakayama M, Kashiwakura J-I, Okayama Y, Kubo M, Ohara O, Kiyono H, Koseki H, Murakami M, Hirano T (2019) Mast cells play role in wound healing through the ZnT2/GPR39/IL-6 axis. Scientific Reports 9(1): 10842. https://doi.org/10.1038/s41598-019-47132-5 [PubMed] [PMC]
Nosbaum A, Prevel N, Truong H-A, Mehta P, Ettinger M, Scharschmidt TC, Ali NH, Pauli ML, Abbas AK, Rosenblum MD (2016) Cutting edge: Regulatory T cells facilitate cutaneous wound healing. Journal of Immunology 196(5): 2010–2014. https://doi.org/10.4049/jimmunol.1502139 [PubMed] [PMC]
O’Dell BL, Reynolds G, Reeves PG (1977) Analogous effects of zinc deficiency and aspirin toxicity in the pregnant rat. The Journal of Nutrition 107(7): 1222–1228. https://doi.org/10.1093/jn/107.7.1222 [PubMed]
Oeckinghaus A, Ghosh S (2009) The NF-kappaB family of transcription factors and its regulation. Cold Spring Harbor Perspectives in Biology 1(4): a000034. https://doi.org/10.1101/cshperspect.a000034 [PubMed] [PMC]
Oyarzun-Ampuero F, Vidal A, Concha M, Morales J, Orellana S, Moreno-Villoslada I (2015) Nanoparticles for the treatment of wounds. Current Pharmaceutical Design 21(29): 4329–4341. https://doi.org/10.3390/molecules23092392[PubMed]
Pati R, Das I, Mehta RK, Sahu R, Sonawane A (2016) Zinc-oxide nanoparticles exhibit genotoxic, clastogenic, cytotoxic and actin depolymerization effects by inducing oxidative stress responses in macrophages and adult mice. Toxicological Sciences 150(2): 454–472. https://doi.org/10.1093/toxsci/kfw010 [PubMed]
Pawlak K, Mysliwiec M, Pawlak D (2012) The alteration in Cu/Zn superoxide dismutase and adhesion molecules concentrations in diabetic patients with chronic kidney disease: the effect of dialysis treatment. Diabetes Research and Clinical Practice 98(2): 264–270. https://doi.org/10.1016/j.diabres.2012.09.012 [PubMed]
Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC (1997) The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. The Journal of Cell Biology 137(6): 1445–1457. https://doi.org/10.1083/jcb.137.6.1445 [PubMed] [PMC]
Posthauer ME (2014) Nutrition: fuel for pressure ulcer prevention and healing. Nursing 44(12): 67–69. https://doi.org/10.1097/01.NURSE.0000456389.22724.ef [PubMed]
Prasad AS (2014) Zinc is an antioxidant and anti-inflammatory agent: Its role in human health. Frontiers in Nutrition 1: 14. https://doi.org/10.3389/fnut.2014.00014 [PubMed] [PMC]
Prasad AS (2020) Lessons learned from experimental human model of zinc deficiency. Journal of Immunology Research 2020: 9207279. https://doi.org/10.1155/2020/9207279 [PubMed] [PMC]
Prasad AS, Bao B, Beck FW, Sarkar FH (2001) Zinc activates NF-kappaB in HUT-78 cells. The Journal of Laboratory and Clinical Medicine 138(4): 250–256. https://doi.org/10.1067/mlc.2001.118108 [PubMed]
Razzaq HAA, Gomez d’Ayala G, Santagata G, Bosco F, Mollea C, Larsen N, Duraccio D (2021) Bioactive films based on barley β-glucans and ZnO for wound healing applications. Carbohydrate Polymers 272: 118442. https://doi.org/10.1016/j.carbpol.2021.118442 [PubMed]
Rohani MG, Parks WC (2015) Matrix remodeling by MMPs during wound repair. Matrix Biology 44–46: 113–121. https://doi.org/10.1016/j.matbio.2015.03.002 [PubMed]
Roohani N, Hurrell R, Kelishadi R, Schulin R (2013) Zinc and its importance for human health: An integrative review. Journal of Research in Medical Sciences 18(2): 144–157. [PubMed] [PMC]
Rosenkranz E, Hilgers R-D, Uciechowski P, Petersen A, Plümäkers B, Rink L (2017) Zinc enhances the number of regulatory T cells in allergen-stimulated cells from atopic subjects. European Journal of Nutrition 56(2): 557–567. https://doi.org/10.1007/s00394-015-1100-1 [PubMed]
Rosenkranz E, Metz CHD, Maywald M, Hilgers R-D, Weßels I, Senff T, Haase H, Jäger M, Ott M, Aspinall R, Plümäkers B, Rink L (2016) Zinc supplementation induces regulatory T cells by inhibition of Sirt-1 deacetylase in mixed lymphocyte cultures. Molecular Nutrition & Food Research 60(3): 661–671. https://doi.org/10.1002/mnfr.201500524[PubMed]
Rousselle P, Braye F, Dayan G (2019) Re-epithelialization of adult skin wounds: Cellular mechanisms and therapeutic strategies. Advanced Drug Delivery Reviews 146: 344–365. https://doi.org/10.1016/j.addr.2018.06.019 [PubMed]
Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, Stiborova M, Adam V, Kizek R (2013) The role of metallothionein in oxidative stress. International Journal of Molecular Sciences 14(3): 6044–6066. https://doi.org/10.3390/ijms14036044 [PubMed] [PMC]
Sabino F, Keller U auf dem (2015) Matrix metalloproteinases in impaired wound healing. Metalloproteinases in Medicine 2: 1–8. https://doi.org/10.2147/MNM.S68420
Satianrapapong W, Pongkorpsakol P, Muanprasat C (2020) A G-protein coupled receptor 39 agonist stimulates proliferation of keratinocytes via an ERK-dependent pathway. Biomedicine & Pharmacotherapy 127: 110160. https://doi.org/10.1016/j.biopha.2020.110160 [PubMed]
Sharma DK, Sharma KK, Kumar V, Sharma A (2016) Effect of Ce doping on the structural, optical and magnetic properties of ZnO nanoparticles. Journal of Materials Science: Materials in Electronics 27: 10330–10335. https://doi.org/10.1007/s10854-016-5117-x
Sheets AR, Demidova-Rice TN, Shi L, Ronfard V, Grover KV, Herman IM (2016) Identification and characterization of novel matrix-derived bioactive peptides: A role for collagenase from Santyl® ointment in post-debridement wound healing? PloS One 11(7): e0159598. https://doi.org/10.1371/journal.pone.0159598 [PubMed] [PMC]
Shembade N, Ma A, Harhaj EW (2010) Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327(5969): 1135–1139. https://doi.org/10.1126/science.1182364 [PubMed] [PMC]
Shi Y, Zou Y, Shen Z, Xiong Y, Zhang W, Liu C, Chen S (2020) Trace elements, PPARs, and metabolic syndrome. International Journal of Molecular Sciences 21(7): 2612. https://doi.org/10.3390/ijms21072612 [PubMed] [PMC]
Sobczak AIS, Pitt SJ, Stewart AJ (2018) Influence of zinc on glycosaminoglycan neutralisation during coagulation. Metallomics 10(9): 1180–1190. https://doi.org/10.1039/c8mt00159f [PubMed] [PMC]
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annual Review of Cell and Developmental Biology 17: 463–516. https://doi.org/10.1146/annurev.cellbio.17.1.463 [PubMed] [PMC]
Taylor KA, Pugh N (2016) The contribution of zinc to platelet behaviour during haemostasis and thrombosis. Metallomics 8(2): 144–155. https://doi.org/10.1039/c5mt00251f [PubMed]
Thingholm TE, Rönnstrand L, Rosenberg PA (2020) Why and how to investigate the role of protein phosphorylation in ZIP and ZnT zinc transporter activity and regulation. Cellular and Molecular Life Sciences 77(16): 3085–3102. https://doi.org/10.1007/s00018-020-03473-3 [PubMed] [PMC]
Tobita Y, Arima T, Nakano Y, Uchiyama M, Shimizu A, Takahashi H (2020) Peroxisome proliferator-activated receptor beta/delta agonist suppresses inflammation and promotes neovascularization. International Journal of Molecular Sciences 21(15): 5296. https://doi.org/10.3390/ijms21155296 [PubMed] [PMC]
Tobita Y, Arima T, Nakano Y, Uchiyama M, Shimizu A, Takahashi H (2021) Effects of selective peroxisome proliferator activated receptor agonists on corneal epithelial wound healing. Pharmaceuticals 14: 88. https://doi.org/10.3390/ph14020088 [PubMed] [PMC]
Vereecke L, Beyaert R, van Loo G (2009) The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends in Immunology 30(8): 383–391. https://doi.org/10.1016/j.it.2009.05.007 [PubMed]
Voelkl J, Tuffaha R, Luong TTD, Zickler D, Masyout J, Feger M, Verheyen N, Blaschke F, Kuro-O M, Tomaschitz A, Pilz S, Pasch A, Eckardt K-U, Scherberich JE, Lang F, Pieske B, Alesutan I (2018) Zinc inhibits phosphate-induced vascular calcification through TNFAIP3-mediated suppression of NF-κB. Journal of the American Society of Nephrology 29(6): 1636–1648. https://doi.org/10.1681/ASN.2017050492 [PubMed] [PMC]
Wang X, Khalil RA (2018) Matrix metalloproteinases, vascular remodeling, and vascular disease. Advances in Pharmacology 81: 241–330. https://doi.org/10.1016/bs.apha.2017.08.002 [PubMed] [PMC]
Wang Y, Ivanov I, Smith SA, Gailani D, Morrissey JH (2019) Polyphosphate, Zn2+ and high molecular weight kininogen modulate individual reactions of the contact pathway of blood clotting. Journal of Thrombosis and Haemostasis 17(12): 2131–2140. https://doi.org/10.1111/jth.14612 [PubMed] [PMC]
Wätjen W, Benters J, Haase H, Schwede F, Jastorff B, Beyersmann D (2001) Zn2+ and Cd2+ increase the cyclic GMP level in PC12 cells by inhibition of the cyclic nucleotide phosphodiesterase. Toxicology 157(3): 167–175. https://doi.org/10.1016/s0300-483x(00)00370-x [PubMed]
Watson BR, White NA, Taylor KA, Howes J-M, Malcor J-DM, Bihan D, Sage SO, Farndale RW, Pugh N (2016) Zinc is a transmembrane agonist that induces platelet activation in a tyrosine phosphorylation-dependent manner. Metallomics 8(1): 91–100. https://doi.org/10.1039/c5mt00064e [PubMed]
Weisel JW, Litvinov RI (2017) Fibrin formation, structure and properties. Sub-Cellular Biochemistry 82: 405–456. https://doi.org/10.1007/978-3-319-49674-0_13 [PubMed] [PMC]
Wessels I, Haase H, Engelhardt G, Rink L, Uciechowski P (2013) Zinc deficiency induces production of the proinflammatory cytokines IL-1β and TNFα in promyeloid cells via epigenetic and redox-dependent mechanisms. The Journal of Nutritional Biochemistry 24(1): 289–297. https://doi.org/10.1016/j.jnutbio.2012.06.007 [PubMed]
Xiong H-M (2013) ZnO nanoparticles applied to bioimaging and drug delivery. Advanced Materials 25(37): 5329–5335. https://doi.org/10.1002/adma.201301732 [PubMed]
Yu M, Lee W-W, Tomar D, Pryshchep S, Czesnikiewicz-Guzik M, Lamar DL, Li G, Singh K, Tian L, Weyand CM, Goronzy JJ (2011) Regulation of T cell receptor signaling by activation-induced zinc influx. The Journal of Experimental Medicine 208(4): 775–785. https://doi.org/10.1084/jem.20100031 [PubMed] [PMC]
Zabel U, Schreck R, Baeuerle PA (1991) DNA binding of purified transcription factor NF-kappa B. Affinity, specificity, Zn2+ dependence, and differential half-site recognition. The Journal of Biological Chemistry 266(1): 252–260. https://doi.org/10.1016/S0021-9258(18)52428-5 [PubMed]
Zhang T, Kuliyev E, Sui D, Hu J (2019) The histidine-rich loop in the extracellular domain of ZIP4 binds zinc and plays a role in zinc transport. The Biochemical Journal 476(12): 1791–1803. https://doi.org/10.1042/BCJ20190108[PubMed] [PMC]
Zheng J, Lang Y, Zhang Q, Cui D, Sun H, Jiang L, Chen Z, Zhang R, Gao Y, Tian W, Wu W, Tang J, Chen Z (2015) Structure of human MDM2 complexed with RPL11 reveals the molecular basis of p53 activation. Genes & Development 29(14): 1524–1534. https://doi.org/10.1101/gad.261792.115 [PubMed] [PMC]
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Research Results in Pharmacology

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

