The influence of the vagus nerve and indole derivative SS-68 on excitation processes in the SA node
DOI:
https://doi.org/10.18413/rrpharmacology.9.10054Abstract
Introduction: Atrial fibrillation (AF) is the most common form of cardiac arrhythmias. Studying the pathogenesis of this pathological process will make it possible to look for new methods of treating AF and to predict its occurrence in a more targeted way. The aim of the study was to identify the components of the takeover process of central rhythmogenesis by the SA node in the conditions of atrial fibrillation when stimulating the vagus nerve and using substance SS-68.
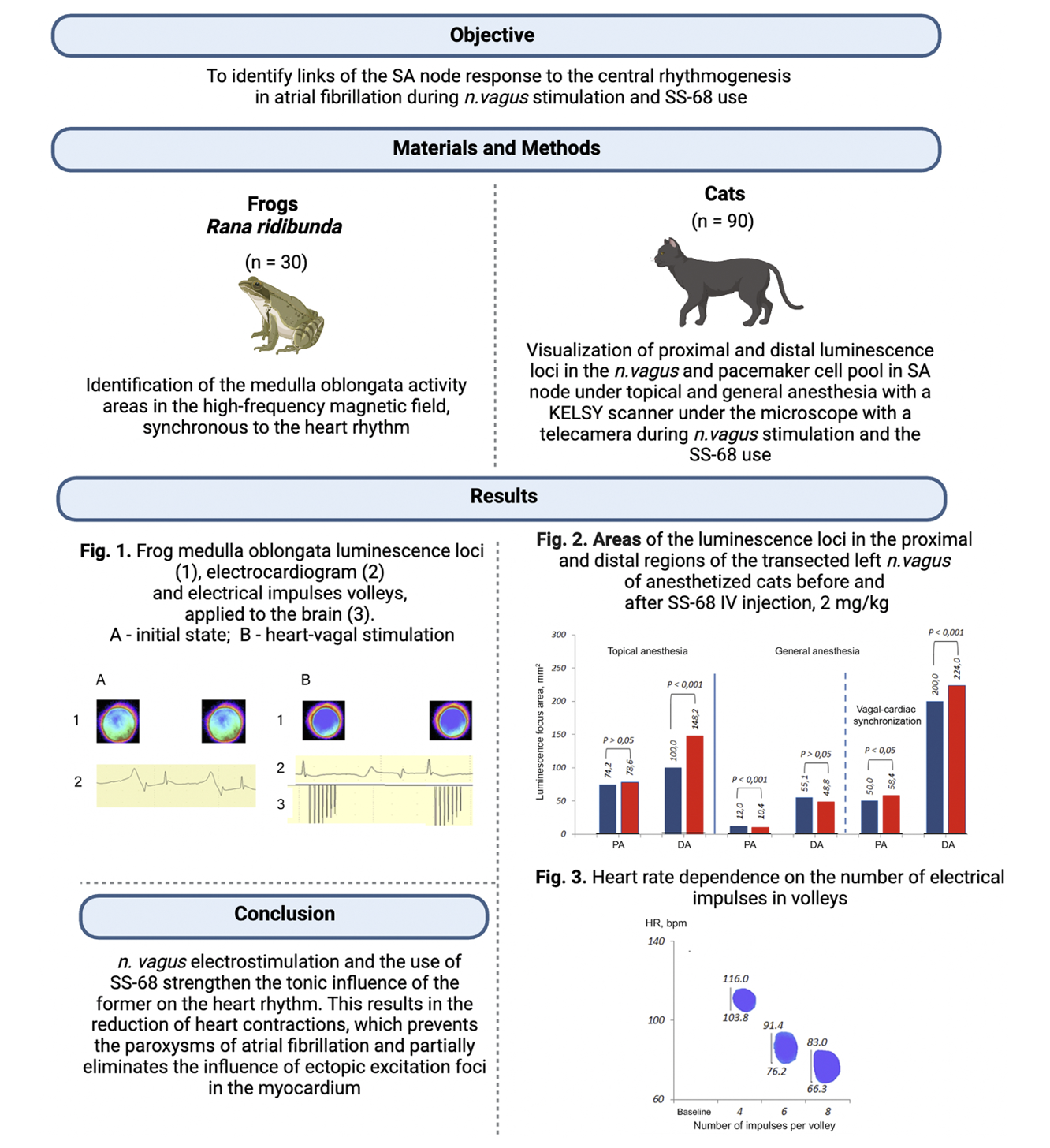
Materials and Methods: The experiments were conducted on 30 frogs and 90 cats. In frogs, the activity of the regions of the medulla oblongata synchronous with the heart rhythm was determined in a high-frequency electromagnetic field. In cats, proximal and distal foci of luminescence in the vagus nerve (VN) and pools of pacemaker cells (PCs) in the sinoatrial node were visualized under topical and general anesthesia, using a KELSY scanner with a microscope video capture unit while stimulating VN and using SS-68.
Results and Discussion: The stimulation of VN with volleys of electrical impulses and the introduction of SS-68 increase the foci of luminescence in the nerve and unite the PC pools. This way, under general anesthesia in comparison with topical anesthesia, the area of the proximal focus of VN luminescence decreased by 83.8%, and the distal focus – by 44.9%. Against the background of general anesthesia, the area of the proximal focus of luminescence when stimulating VN with volleys of electrical impulses was by 76.0% larger than before stimulation, and the distal focus – by 72.5%. After the administration of SS-68, there was an increase in the foci of luminescence: under general anesthesia, when compared with topical anesthesia, the area of the proximal foci of luminescence decreased by 86.8%, and the distal one – by 67.1%. Under general anesthesia, the area of the proximal focus of luminescence under conditions of stimulating VN with volleys of electrical impulses was by 82.2% larger than before stimulation and the distal one – by 78.2%. When signals from the brain arrive simultaneously through VN at the PC pools, they are absorbed by the PC pools; the focus of early depolarization becomes wide, which prevents the development of AF. The increased synchronizing influence of VN may be one of the methods for treating autonomic AF, and if its influence decreases, it can be a prognostic factor for the occurrence of recurrent AF.
Conclusion: The tonic effect of VN on the heart rhythm through electrical stimulation of the former and the use of SS-68 is manifested in a decreased heart rate: the difference between the initial heart rhythm and the minimal synchronization range boundary. A decrease in the heart rate under the influence of VN prevents paroxysms of AF, but does not completely eliminate the influence of ectopic foci on it.
Graphical Abstract
Keywords:
substance SS-68, pacemaker cells, sinoatrial node, brain rhythmReferences
Abramochkin DV, Sukhova GS, Rozenshtraukh LV (2009) Mechanisms of functioning and regulation of the sinoatrial node in mammals. Advances in Physiological Sciences [Uspekhi Fiziologicheskikh Nauk] 40(4): 21–41. [in Russian]
Ardell JL (2011) The cardiac neuronal hierarchy and susceptibility to arrhythmias. Heart Rhythm 8: 590–591. http://doi.org/10.1016/j.hrthm.2010.12.019 [PubMed] [PMC]
Baruscotti M, Robinson RB (2007) Electrophysiology and pacemaker function of the developing sinoatrial node. The American Journal of Physiology: Heart and Circulatory Physiology 293(5): 2613–2623. https://doi.org/10.1152/ajpheart.00750.2007. [PubMed]
Bradd AD, Al Abed A, Guo T, Lovell NH, Dokos S (2012) Study of cardiac pacemaker excitation using generic ionic models and realistic cell distribution. Annual International Conference of the IEEE Engineering in Medicine and Biology Society 2012: 195–198. https://doi.org/10.1109/EMBC.2012.6345904 [PubMed]
Brotman DJ, Golden SH, Wittstein IS (2007) The cardiovascular toll of stress. Lancet 370(9592): 1089–1100. http://doi.org/10.1016/S0140-6736(07)61305-1 [PubMed]
Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S (2014) Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circulation Research 114(9): 1500–1515. http://doi.org/10.1161/CIRCRESAHA.114.303772 [PubMed] [PMС]
Chen PS, Tan AY (2007) Autonomic nerve activity and atrial fibrillation. Heart Rhythm 4: S61–S64. http://doi.org/10.1016/j.hrthm.2006.12.006 [PubMed] [PMC]
Chernyavsky AM, Rakhmonov SS, Pak IA, Kareva YuE (2013) The role of the autonomic nervous system in the development of atrial fibrillation. Clinical Medicine [Klinicheskaya Meditsina] 91(1): 16–20. [in Russian]
Filatov AG, Tarashvili EG (2012) Epidemiology and social significance of atrial fibrillation. Annals of Arrhythmology [Annaly Aritmologii] 2: 5–14. [in Russian]
Filyukova MV (2018) Sinus node dysfunction. Journal of Basic Medicine and Biology [Zhurnal Fundamental’nou Meditsiny i Biologii] 1: 11–19. [in Russian]
Galenko-Yaroshevsky PA, Alekseenko SN, Kanorsky SG, Bogus S, Dukhanin AS, Vasiliev PM, Galenko-Yaroshevsky AP, Pavlyuchenko II, Lebedeva SA, Nadein KA, Nechepurenko AA, Suzdalev KF, Zelenskaya AV, Uvarov AV (2023) Atrial fibrillation. Status of the problem and prospects for creating a new antifibrillator agent based on indole amino derivatives. Prosveshchenie-Yug, Krasnodar, 747 pp. [in Russian]
Garg PK, O'Neal WT, Diez‐Roux AV, Alonso A, Soliman EZ, Heckbert S (2019) Negative affect and risk of atrial fibrillation. MESA. Journal of American Heart Association 8(1): e01060. http://doi.org/10.1161/JAHA.118.010603[PubMed] [PMC]
Grigoriev MG, Babich LN (2015) Model of excitation of pacemaker cardiomyocytes of the cardiac conduction system. Young Scientist [Molodoi Ucheny] 10(90): 178–184. [in Russian]
Karemaker JM (2015) How the vagus nerve produces beat-to-beat heart rate variability; experiments in rabbits to mimic in vivo vagal patterns. Journal Clinical of Translational Research 1(3): 190–204. [PubMed] [PMC]
Legallois D, Gomes S, Pellissier A, Milliez P (2013) Medical emotional stress-induced atrial fibrillation: My own personal experience. International Journal of Cardiology 167(6): 182–183. http://doi.org/10.1016/j.ijcard.2013.04.074[PubMed]
Linz D, Ukena C, Mahfoud F, Neuberger HR, Böhm M (2014) Atrial autonomic innervation: a target for interventional antiarrhythmic therapy. Journal of the American College Cardiology 63(3): 215–224. http://doi.org/10.1016/j.jacc.2013.09.020 [PubMed]
Lorincz I, Szabó Z, Simkó J, Szánthó E, Barta K, Füzi M, Szigeti G. (2008) Atrial fibrillation and the autonomous nervous system. Orvosi Hetilap 149(43): 2019–2028. http://doi.org/10.1556/OH.2008.28466 [PubMed] [in Hungarian]
Mazurov ME (2006) Rhythmogenesis in the sinoatrial unit of the heart. Biofizika 51(6): 1092–1099. [PubMed]
Mazurov ME (2009) Control of the unified heart rhythm. Biofizika 54(1): 89-96. [PubMed]
Mironova TF, Mironov VA, Obukhova TYu, Shmonina OG, Mordas EYu, Kudrina KS, Milovankina NO, Milashchenko AI (2018) Autonomic regulation of heart rate (review). Ural Medical Journal [Ural’sky Meditsinsky Zhurnal] 10(165): 90–105. [in Russian]
Murphy C, Lazzara R (2016) Current concepts of anatomy and electrophysiology of the sinus node. Journal of Interventional Cardiac Electrophysiology 46(1): 9–18. https://doi.org/10.1007/s10840-016-0137-2. [PubMed]
Nechepurenko AA, Romantsov EI (2020) The influence of the vagus nerve on the duration of paroxysmal atrial fibrillation and the zone of early depolarization in the sinoatrial region of the heart. In: Higher School: Scientific Research. Proceedings of the Interuniversity Scientific Congress, Moscow, Russia, February 19, 2020. Infinity Publishing House, Ufa, p. 136–143. [in Russian]
Pokrovsky VM (2007) Formation of heart rhythm in humans and animals. Kuban-kniga Publishing House, Krasnodar, 143 pp. [in Russian]
Pokrovsky VM, Nechepurenko AA, Tarasov DG, Korotkov KG, Abushkevich VG (2019) Sinoatrial node pacemaker cells pool Dynamics upon synchronization with vagus nerve rhythm. Journal of Applied Biotechnology and Bioengineering 6(3): 114–116. https://doi.org/10.15406/jabb.2019.06.00182
Pokrovsky VM, Tarasov DG, Nechepurenko AA, Korotkov KG, Abushkevich VG (2018) The focus of early depolarization of the sinoatrial region of the right atrium in humans before anesthesia and during anesthesia. Clinical and Experimental Surgery [Klinicheskaya n Eksperimental’naya Khirurgiya]. Journal named after academician BV Petrovsky 6(4 (22)): 49–54. https://doi.org/10.24411/2308-1198-2018-14007 [in Russian]
Svensson T, Kitlinski M, Engström G, Melander O (2017) Psychological stress and risk of incident atrial fibrillation in men and women with known atrial fibrillation genetic risk scores. Scientific Reports 7: 42613. http://doi.org/10.1038/srep42613 [PubMed] [PMC]
Van Weerd JH, Christoffels VM (2016) The formation and function of the cardiac conduction system. Development 143: 197210. https://doi.org/10.1242/dev.124883 [PubMed]
Vislobokov AI, Bogus SK, Ignatov YuD, Galenko-Yaroshevsky PA, Melnikov KN (2012) Comparative membranotropic activity of indole derivative SS-68 and amiodarone on mollusk neurons. New Technologies [Novye Tekhnologii] 4: 283–290. [in Russian]
Yaniv Y, Ahmet I, Liu J, Lyashkov AE, Guiriba T, Okamoto Y, Ziman BD, Lakatta EG (2014) Synchronization of sinoatrial node pacemaker cell clocks and its autonomic modulation impart complexity to heart beating intervals. Heart Rhythm 11(7): 1210–1219. http://doi.org/10.1016/j.hrthm.2014.03.049 [PubMed] [PMC]
Yaniv Y, Lyashkov AE, Lakatta EG (2014a) Impaired signaling intrinsic to sinoatrial node pacemaker cells affects heart rate variability during cardiac disease. Journal Clinical of Trials 4(1): 152. http://doi.org/10.4172/2167-0870.1000152. [PubMed] [PMC]
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Anatoliy A. Nechepurenko, Pavel A. Galenko-Yaroshevsky, Vladimir M. Pokrovskiy, Anait V. Zelenskaya, Konstantin F. Suzdalev, Svetlana A. Lebedeva, Natalia M. Makhnova, Alexandr V. Maksemyuk, Ivan A. Minenko, Roman V. Nikitin, Valeriy G. Abushkevich†

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

