Effect of cyclophosphamide on regulation of heart contractions by means of sodium calcium exchanger
DOI:
https://doi.org/10.18413/rrpharmacology.11.539Abstract
Introduction: Every year brings in new medications capable to slow or stop proliferation of tumour cells. Unfortunately, in spite of antitumour benefits, new medicines have some side effects that reduce their therapeutic properties.
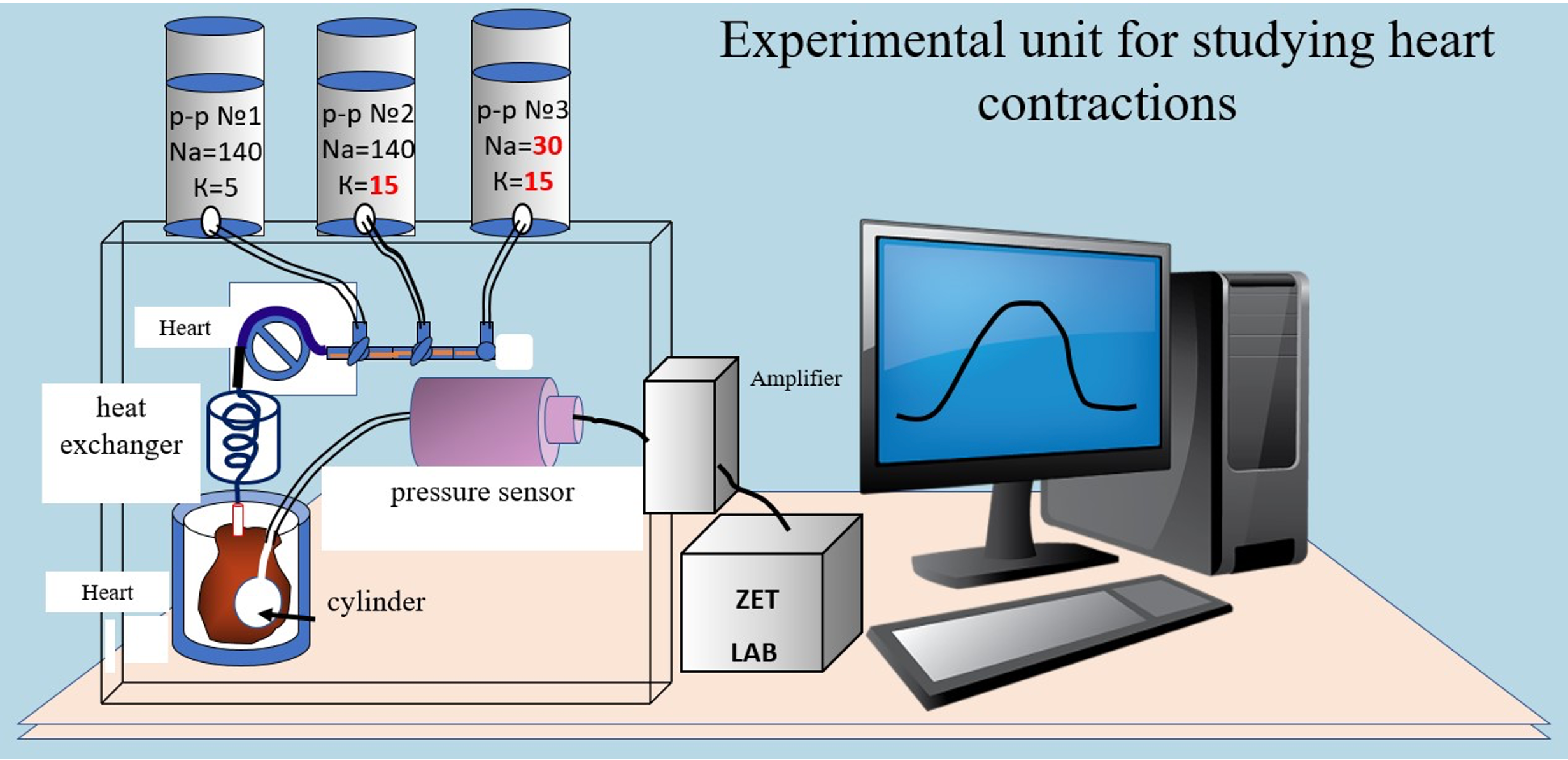
Materials and Methods: The study was conducted in three series of experiments on 24 whiteWistar rats, both male and female, each weighing 200-250 g. Decapitation of the animal was performed under ether anesthesia with rapid extraction of the heart and perfusion using the Langendorff method. To study NCX in the heart, a perfusion device was used to create a permanent coronary flow. Monitoring the physiological state of the heart when changing the composition of solutions was carried out using a balloon inserted into the left ventricle.Contractions and relaxation of the heart were recorded using an electronic pressure sensor. The parameters were documented and processed using the Zet Lab external module software.
Results and Discussion: In the first series of experiments, the effect of hyposodic solution onthe tone of the left ventricle of the heart stopped by a hyperpotassic medium was studied. The developed technique served as the basis for studying the effect of cyclophosphamide on NCX,accompanied by contraction and relaxation of the heart. Experiments have shown the ability of cyclophosphamide to significantly reduce the rate of the tone increase and the development of contraction force, as well as prolong the relaxation time during NCX.
Conclusion: Cyclophosphamide is able to disrupt the capture of ionized calcium in the cytosolby intracellular Ca-accumulating structures during relaxation of the heart. Unlike controlrecordings, in the presence of cyclophosphamide, repeated relaxations do not occur completely.As a result, each subsequent contraction begins at a higher initial diastole level.
Graphical Abstract
Keywords:
NCX, slow calcium channels, sarcoplasmic reticulum, cyclophosphamide, isolated rat’s heart contractionsReferences
Abulfadl Ya, Abo El Ela Y, Alkhaiyat A, Elkhodary I (2023) Cyclophosphamide enfeebles myocardial isometric contraction force via RIP1/RIP3/MLKL/TRPM7-mediated necroptosis. Biomedicine & Pharmacother 163: 114819. https://doi.org/10.1016/j.biopha.2023.114819[PubMed]
Al-Khannaq M, Lytton J (2022) Regulation of K+-dependent Na+/Ca2+-exchangers (NCKX). International Journal of Molecular Sciences 24(1): 598. https://doi.org/10.3390/ijms24010598[PubMed] [PMC]
Bomfim G, Mitaishvili E, Schnetkamp P, Lacruz R (2024) Na+/Ca2+ exchange in enamel cells is dominated by the K+-dependent NCKX exchanger. The Journal of General Physiology 156(1): e202313372. https://doi.org/10.1085/jgp.202313372 [PubMed] [PMC]
Ferreira de Souza T, Quinaglia T, Neilan T, Coelho-Filho O (2019) Assessment of cardiotoxicity of cancer chemotherapy: The value of cardiac MR imaging. Magnetic Resonance Imaging Clinics of North America 27(3): 533–544. https://doi.org/10.1016/j.mric.2019.04.001 [PubMed] [PMC]
Flenner F, Arlt N, Nasib M, Schobesberger S, Koch Th, Ravens U, Friedrich F, Nikolaev V, Christ T, Stehr S (2018) In vitro negative inotropic effect of low concentrations of bupivacaine relates to diminished Ca2+ sensitivity but not to Ca2+ handling or β-adrenoceptor signaling. Anesthesiology 128(6): 1175–1186. https://doi.org/10.1097/ALN.0000000000002180 [PubMed]
Frolova OG, Gladchenko MP, Artyushkova EB, Khvostovoy VV, Kolesnikova AM, Anurova EV, Chernyatina MA (2020) Experimental substantiation of promising ways to reduce the cardiotoxicity of cyclophosphamide using an immobilized form of cyclophosphamide and a cytoprotector. Modern Problems of Science and Education [Sovremennye Problemy Nauki i Obrazovaniya] 5. https://doi.org/10.17513/spno.30087 [in Russian]
Gorvin C (2019) Molecular and clinical insights from studies of calcium-sensing receptor mutations. Journal of Molecular Endocrinology 63(2): R1–R16. https://doi.org/10.1530/JME-19-0104 [PubMed]
Hilgemann D (2020) Control of cardiac contraction by sodium: Promises, reckonings, and new beginnings. Cell Calcium 85: 102129. https://doi.org/10.1016/j.ceca.2019.102129 [PubMed] [PMC]
Liu Ch-H, Chen Z, Oliva M, Luo J, Collier S, Montell C, Hardie R (2020) Rapid Release of Ca2+ from endoplasmic reticulum mediated by Na+/Ca2+ exchange. The Journal of Neuroscience 40(16): 3152–3164. https://doi.org/10.1523/JNEUROSCI.2675-19.2020 [PubMed] [PMC]
Mourouzis I, Kounatidis D, Brozou V, Anagnostopoulos D, Katsaouni A, Lourbopoulos A, Pantos C (2023) Effects of T3 administration on ex vivo rat hearts subjected to normothermic perfusion: Therapeutic implications in donor heart preservation and repair. Transplant International 36: 10742. https://doi.org/10.3389/ti.2023.10742 [PubMed] [PMC]
O'Donnell P, Jones R (2023) The development of post-transplant cyclophosphamide: Half a century of translational team science. Blood Review 62: 101034. https://doi.org/10.1016/j.blre.2022.101034 [PubMed] [PMC]
O'Halloran D (2020) Simulation model of CA1 pyramidal neurons reveal opposing roles for the Na+/Ca2+ exchange current and Ca2+-activated K+ current during spike-timing dependent synaptic plasticity. Plos One 15(3): e0230327. https://doi.org/10.1371/journal.pone.0230327[PubMed] [PMC]
Taslimi P, Kandemir F, Demir Y, İleritürk M, Temel Y, Caglayan C, Gulçin İ (2019) The antidiabetic and anticholinergic effects of chrysin on cyclophosphamide-induced multiple organ toxicity in rats: Pharmacological evaluation of some metabolic enzyme activities. Journal of Biochemical and Molecular Toxicology 33(6): e22313. https://doi.org/10.1002/jbt.22313 [PubMed]
Xue J, Zeng W, Han Y, John S, Ottolia M, Jiang Y (2023) Structural mechanisms of the human cardiac sodium-calcium exchanger NCX1. Nature Communications 14(1): 6181.https://doi.org/10.1038/s41467-023-41885-4 [PubMed] [PMC]
Yue X, Hazan A, Lotteau S, Zhang R, Torrente A, Philipson K, Ottolia M, Joshua I (2020) Goldhaber Na/Ca exchange in the atrium: Role in sinoatrial node pacemaking and excitation-contraction coupling. Cell Calcium 87: 102167. https://doi.org/10.1016/j.ceca.2020.102167[PubMed] [PMC]
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Татьяна А. Бережнова, Иван П. Мошуров, Ян Баофен, Чаоцянь Сюй; Ирина В. Коваленко; Владимир В. Алабовский, Алексей А. Винокуров, Олег В. Маслов, Яна В. Кулинцова

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

