Regulation of 11β-hydroxysteroid dehydrogenase isoforms – novel drug targets for osteoporosis therapy
DOI:
https://doi.org/10.18413/rrpharmacology.11.807Abstract
Introduction: Osteoporosis is an significant medical and social public health problem in an aging or elderly society, the issue of pharmacological correction of which remains unresolved to this day.
Materials and Methods: The rationale for this idea stems from our previous findings on the role of 11B-HSD type 2 in bone remodeling and osteoreparation, combined with a content analysis and literature review of scientific publications from PubMed, Scopus, Cyberleninka, Google Scholar, and ResearchGate.
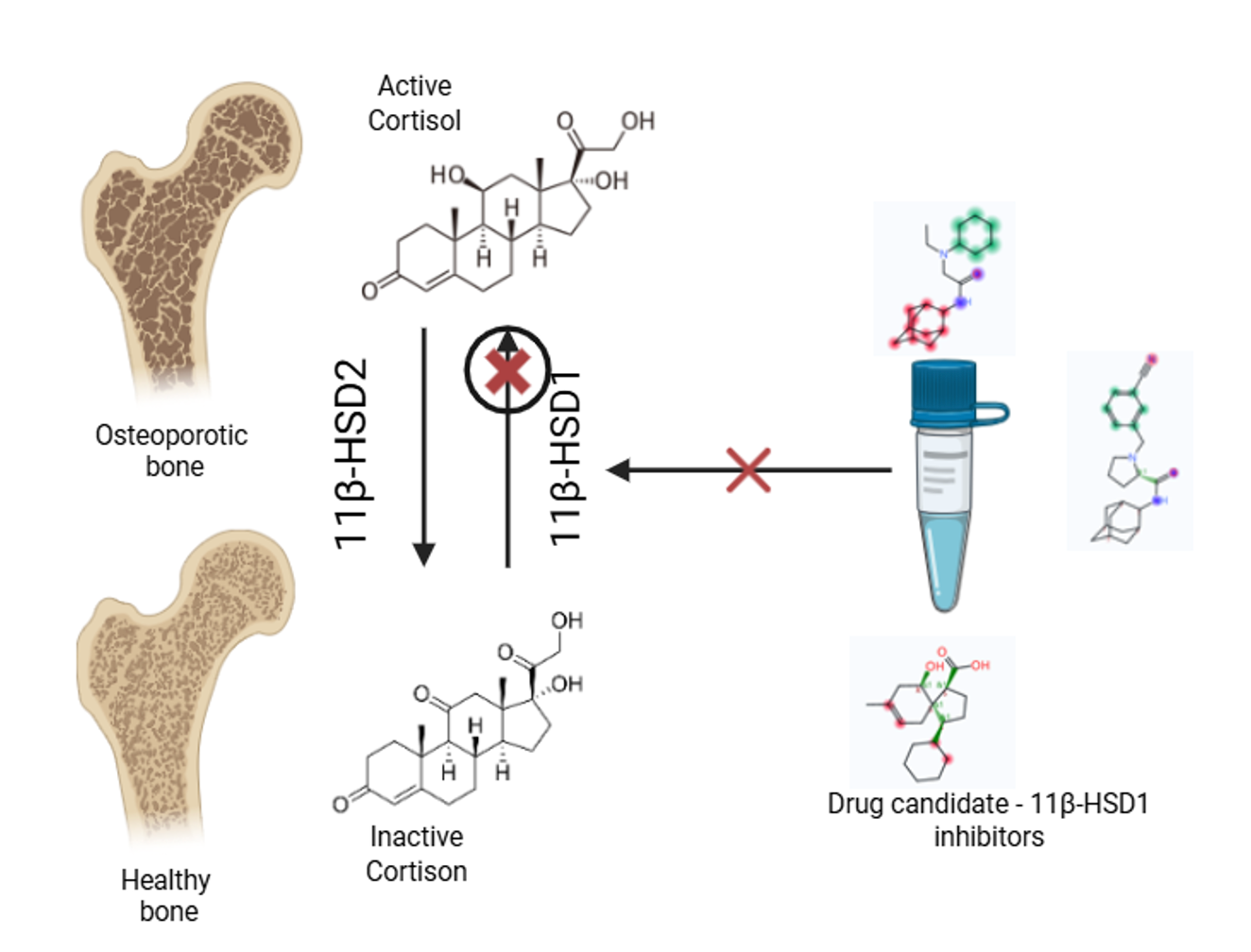
Results and Discussion: Current understanding of the molecular mechanisms of bone homeostasis allows for a significant shift and expansion in the paradigms for treating and preventing osteoporosis. 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) is a key metabolic enzyme that catalyzes the intracellular conversion of inactive glucocorticoids into physiologically active ones. Research conducted over the past decade has shown that abnormal 11β-HSD1 activity contributes to the pathogenesis of obesity, type 2 diabetes, metabolic syndrome, and osteoporosis. The scientific challenge of regulating the activity of 11β-hydroxysteroid dehydrogenase (11β-HSD) isoforms and restoring homeostasis in the 11β-HSD1/11β-HSD2 enzymatic system is proposed to be addressed through the design and application of novel azole-based heterocyclic compounds as 11β-HSD1 inhibitors.
Conclusion: The development of azole-based heterocyclic 11β-HSD1 inhibitors is expected to yield promising drug candidates for pharmacologically correcting impaired bone remodeling and repair.
Graphical Abstract
Keywords:
osteoporosis, 11β-hydroxysteroid dehydrogenase, regulation, 11β-HSD inhibitors, drug targetsReferences
Chapman K, Holmes M, Seckl J (2013) 11Β-Hydroxysteroid dehydrogenases intracellular gate-keepers of tissue glucocorticoid action. Physiological Reviews 93(3): 1139–1206. https://doi.org/10.1152/physrev.00020.2012 [PubMed] [PMC]
Frenkel B, White W, Tuckermann J (2015) Glucocorticoid-induced osteoporosis. Advances in Experimental Medicine and Biology 872: 179–215. https://doi.org/10.1007/978-1-4939-2895-8_8 [PubMed] [PMC]
Garcia J, Smith SS, Karki S, Drissi H, Hrdlicka HH, Youngstrom DW, Delany AM (2021) miR-433-3p suppresses bone formation and mRNAs critical for osteoblast function in mice. Journal of Bone and Mineral Research 36(9): 1808–1822. https://doi.org/10.1002/jbmr.4339 [PubMed]
Korokin MV, Gudyrev OS, Lebedev PR, Kochkarov AA, Pokrovskaya TG, Danilenko LM, Zhernakova NI, Avtina TV, Pribylov SA, Parshina AV, Kuzubova EV, Radchenko AI, Koklin IS, Taran EI (2023) Features of bone remodeling and osteoreparation processes in modeling femoral fracture in genetically modified mice with impaired enzymatic regulation of steroid hormone metabolism. Research Results in Pharmacology 9(4): 113–123. https://doi.org/10.18413/rrpharmacology.9.10062
Korokin MV, Soldatov VO, Gudyrev OS (2022) The role of cortisol metabolism in the realization of pathogenetic links in the development of osteoporosis – the rationale for the search for new pharmacotherapeutic targets (review). Research Results in Biomedicine. 8(4): 457–473. https://doi.org/10.18413/2658-6533-2022-8-4-0-5 [in Russian]
Kuipers AL, Kammerer CM, Pratt JH, Bunker CH, Wheeler VW, Patrick AL, Zmuda JM (2016) Association of circulating renin and aldosterone with osteocalcin and bone mineral density in African ancestry families. Hypertension 67(5): 977–982. https://doi.org/10.1161/HYPERTENSIONAHA.115.06837 [PubMed] [PMC]
Kupczyk D, Studzińska R, Kołodziejska R, Baumgart S, Modrzejewska M, Woźniak A (2022) 11β-Hydroxysteroid dehydrogenase type 1 as a potential treatment target in cardiovascular diseases. Journal of Clinical Medicine 11(20): 6190. https://doi.org/10.3390/jcm11206190 [PubMed] [PMC]
Othonos N, Pofi R, Arvaniti A, White S, Bonaventura I, Nikolaou N, Moolla A, Marjot T, Stimson RH, van Beek AP, van Faassen M, Isidori AM, Bateman E, Sadler R, Karpe F, Stewart PM, Webster C, Duffy J, Eastell R, Gossiel F, Cornfield T, Hodson L, Jane Escott K, Whittaker A, Kirik U, Coleman RL, Scott CAB, Milton JE, Agbaje O, Holman RR, Tomlinson JW (2023) 11β-HSD1 inhibition in men mitigates prednisolone-induced adverse effects in a proof-of-concept randomised double-blind placebo-controlled trial. Nature Communications 14(1): 1025. https://doi.org/10.1038/s41467-023-36541-w [PubMed] [PMC]
Park JS, Bae SJ, Choi SW, Son YH, Park SB, Rhee SD, Kim HY, Jung WH, Kang SK, Ahn JH, Kim SH, Kim KY (2014) A novel 11β-HSD1 inhibitor improves diabesity and osteoblast differentiation. Journal of Molecular Endocrinology 52(2): 191–202. https://doi.org/10.1530/JME-13-0177 [PubMed]
Sobolev MS, Faitelson AV, Gudyrev OS, Rajkumar DSR, Dubrovin GM, Anikanov AV, Chernomortseva ES (2018) Study of endothelio- and osteoprotective effects of combination of rosuvastatin with L-norvaline in experiment. Journal of Osteoporosis 2018: 1585749. [PubMed] [PMC]
Xiao H, Wu Z, Li B, Shangguan Y, Stoltz JF, Magdalou J, Chen L, Wang H (2020) The low-expression programming of 11β--HSD2 mediates osteoporosis susceptibility induced by prenatal caffeine exposure in male offspring rats. British Journal of Pharmacology 177(20): 4683–4700. https://doi.org/10.1111/bph.15225 [PubMed] [PMC]
Zhu Y, Olson SH, Hermanowski-Vosatka A, Mundt S, Shah K, Springer M, Thieringer R, Wright S, Xiao J, Zokian H, Balkovec JM (2008) 4-Methyl-5-phenyl triazoles as selective inhibitors of 11β--hydroxysteroid dehydrogenase type I. Bioorganic & Medicinal Chemistry Letters 18(11): 3405–3411. https://doi.org/10.1016/j.bmcl.2008.04.013[PubMed] [PMC]
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Koklin IS, Lebedev PR, Kochkarov AA, Gudyrev OS, Gureev VV, Dolzhikov AA, Taran EI, Korokin MV

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

