Assessment of physiological parameters in the application of a double adeno-associated virus 9 with a codon-optimized DYSF gene for limb girdle muscular dystrophy type R2
DOI:
https://doi.org/10.18413/rrpharmacology.11.642Abstract
Introduction: Gene therapy for Myoshi myopathy is extremely relevant, as it may become the first pathogenetic treatment for dysferlinopathy. The aim of this study was to study the efficacy and safety of the use of a genetic construct, the AAV9-DYSF-DV3’ virus, for the treatment of limb girdle muscular dystrophy LGMD) type R2.
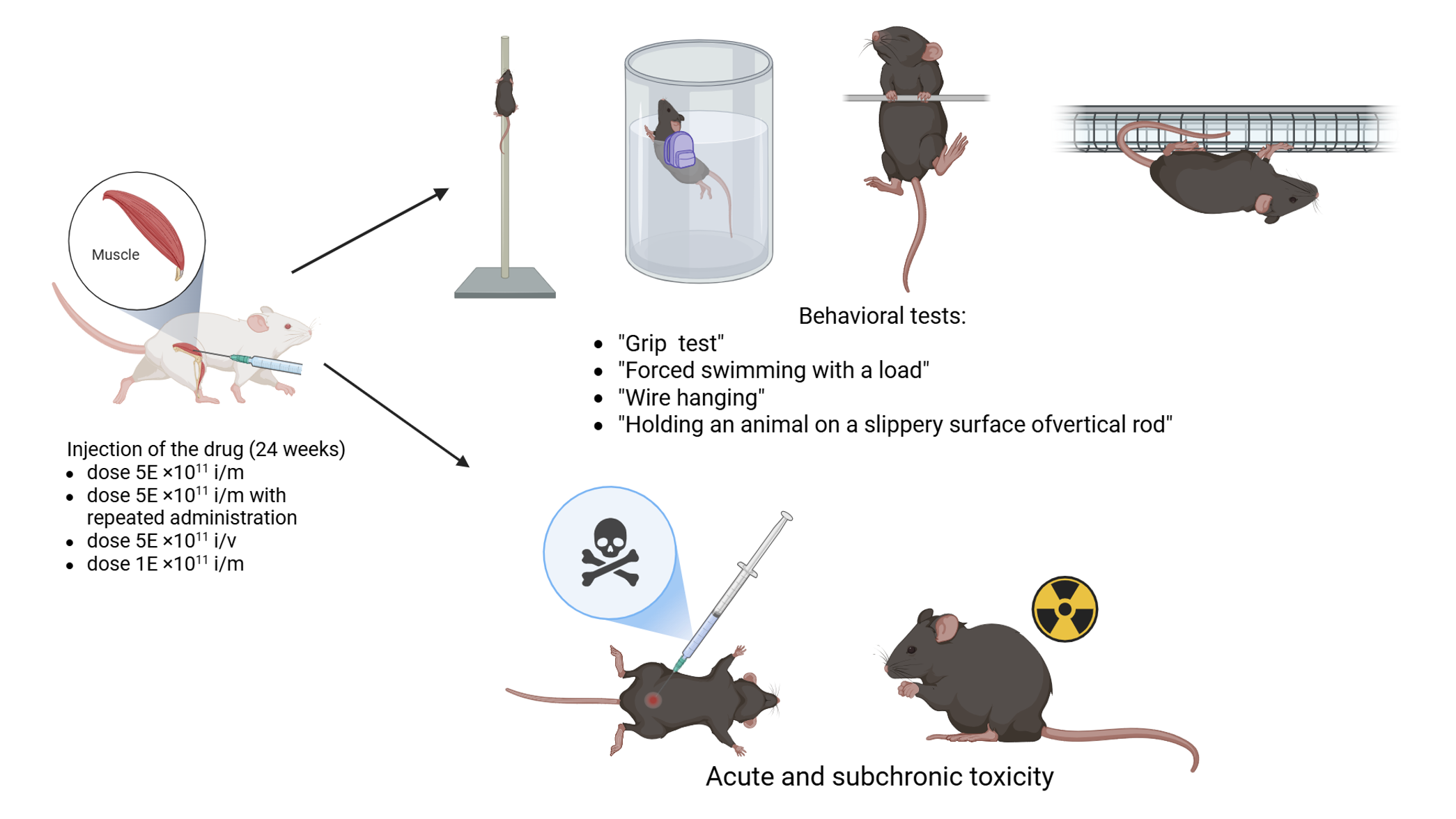
Materials and Methods: Mouse models of limb girdle muscular dystrophy type R2 В6.А-Dysfprmd/GeneJ were used to study the effectiveness of AAV9.DYSF drug and the corresponding C57BL/6J controls were used. During the study, muscle activity was determined on the basis of the following tests: “Grip test”, “Holding an animal on a slippery surface of a vertical rod”, “Forced swimming with a load”, and ”Wire hanging”. In the course of acute and subchronic toxicity, hematological and biochemical blood tests of the rats, histological analysis and ”Open field” behavioral testing were performed.
Results and Discussion: In this study, for the first time, a comprehensive investigation of the effectiveness of gene therapy using the two-vector system of adeno-associated AAV9-DYSF-DV3’ virus with overlapping DYSF cDNA sequences was conducted in a mouse model of limb girdle muscular dystrophy type 2 R.
Conclusion: During the testing of the drug’s effectiveness, it was discovered that drug AAV9.DYSF showed the best effectiveness in mice with the absence of the protein dysferlin in behavioral testing at the maximum dose (5*1012) with a double intramuscular injection. In the “Grip test”, the index in В6.А-Dysfprmd/GeneJ mice increased by 29% (p=0.0026) relative to that in the K-group. In the tests “Forced swimming with a load”, ”Wire hanging”, and ”Holding an animal on a slippery surface of a vertical rod”, the indicators also improved by 80% (p=0.0019), 104.8% (p=0.001) and 20% (p=0.025), respectively, relative to those of the negative control. During acute and subchronic toxicity, the administration of the drug to animals does not cause death or intoxication.
Graphical Abstract
Keywords:
dysferlin, LGMD R2, dual AAV vector, gene transfer, muscle regenerationReferences
Begam M, Collier AF, Mueller AL, Roche R, Galen SS, Roche JA (2018) Diltiazem improves contractile properties of skeletal muscle in dysferlin-deficient BLAJ mice, but does not reduce contraction-induced muscle damage. Physiological Reports 6(11): e13727. https://doi.org/10.14814/phy2.13727 [PubMed] [PMC]
Berezhnova TA, Shishkina VV, Esaulenko DI, Lunyova YA, Goryushkina YS, Abramyan AA, Dyadina KS, Chechelnitsky AI, Samoilenko TV (2023) Combined use of pulse therapy and molecular hydrogen in rats with experimental rheumatoid arthritis: Clinical and histological evaluation of therapeutic effectiveness. Research Results in Pharmacology 9(2): 27–35. https://doi.org/10.18413/rrpharmacology.9.10024
Bouchard C, Tremblay JP (2023) Portrait of dysferlinopathy: Diagnosis and development of therapy. Journal of Clinical Medicine 12(18): 6011. https://doi.org/10.3390/jcm12186011 [PubMed] [PMC]
Fanin M, Angelini C (2016) Progress and challenges in diagnosis of dysferlinopathy. Muscle Nerve. 54(5): 821–835. https://doi.org/10.1002/mus.25367 [PubMed]
García-Campos P, Báez-Matus X, Jara-Gutiérrez C, Paz-Araos M, Astorga C, Cea LA, Rodríguez V, Bevilacqua JA, Caviedes P, Cárdenas AM (2020) N-acetylcysteine reduces skeletal muscles oxidative stress and improves grip strength in dysferlin-deficient bla/j mice. International Journal of Molecular Sciences 21(12): 4293. https://doi.org/10.3390/ijms21124293 [PubMed] [PMC]
Khodabukus A, Prabhu NK, Roberts T, Buldo M, Detwiler A, Fralish ZD, Kondash ME, Truskey GA, Koves TR, Bursac N (2024) Bioengineered model of human LGMD2B skeletal muscle reveals roles of intracellular calcium overload in contractile and metabolic dysfunction in dysferlinopathy. Advanced Science 11(31): e2400188. https://doi.org/10.1002/advs.202400188 [PubMed] [PMC]
Korokin MV, Kuzubova EV, Radchenko AI, Deev RV, Yakovlev IA, Deikin AV, Zhunusov NS, Krayushkina AM, Pokrovsky VM, Puchenkova OA, Chaprov KD, Ekimova NV, Bardakov SN, Chernova ON, Emelin AM, Limaev IS (2022) В6.А-DYSFPRMD/GENEJ mice as a genetic model of dysferlinopathy. Pharmacy & Pharmacology 10(5): 483–496. https://doi.org/10.19163/2307-9266-2022-10-5-483-496
Moore U, Gordish H, Diaz-Manera J, James MK, Mayhew AG, Guglieri M, Fernandez-Torron R, Rufibach LE, Feng J, Blamire AM, Carlier PG, Spuler S, Day JW, Jones KJ, Bharucha-Goebel DX, Salort-Campana E, Pestronk A, Walter MC, Paradas C, Stojkovic T, Mori-Yoshimura M, Bravver E, Pegoraro E, Lowes LP, Mendell JR, Bushby K, Straub V; Jain COS (2021) Consortium. Miyoshi myopathy and limb girdle muscular dystrophy R2 are the same disease. Neuromuscular Disorders 31(4): 265–280. https://doi.org/10.1016/j.nmd.2021.01.009 [PubMed]
Nagaraju K, Raben N, Loeffler L, Parker T, Rochon PJ, Lee E, Danning C, Wada R, Thompson C, Bahtiyar G, Craft J, Hooft Van Huijsduijnen R, Plotz P (2000) Conditional upregulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositisspecific autoantibodies. Proceedings of the National Academy of Sciences of the United States of America 97(16): 9209–9214. https://doi.org/10.1073/pnas.97.16.9209 [PubMed] [PMC]
Nagy N, Nonneman RJ, Llanga T, Dial CF, Riddick NV, Hampton T, Moy SS, Lehtimäki KK, Ahtoniemi T, Puoliväli J, Windish H, Albrecht D, Richard I, Hirsch ML (2017) Hip region muscular dystrophy and emergence of motor deficits in dysferlin-deficient Bla/J mice. Physiological Reports 5(6): e13173. https://doi.org/10.14814/phy2.13173 [PubMed] [PMC]
Park HJ, Hong YB, Hong JM, Yun U, Kim SW, Shin HY, Kim SM, Choi YC (2021) Null variants in DYSF result in earlier symptom onset. Clinical Genetics 99(3): 396–406. https://doi.org/10.1111/cge.13887 [PubMed]
Patel NJ, Van Dyke KW, Espinoza LR (2017) Limb-girdle muscular dystrophy 2B and Miyoshi presentations of dysferlinopathy. The American Journal of the Medical Sciences 353(5): 484–491. https://doi.org/10.1016/j.amjms.2016.05.024 [PubMed]
Patel RB, Dhanesha N, Sutariya B, Ghatge M, Doddapattar P, Barbhuyan T, Kumskova M, Leira EC, Chauhan AK (2023) Targeting neutrophil α9 improves functional outcomes after stroke in mice with obesity-induced hyperglycemia. Stroke 54(9): 2409–2419. https://doi.org/10.1161/STROKEAHA.123.042714 [PubMed] [PMC]
Polikarpova AV, Egorova TV, Bardina MV (2022) Genetically modified animal models of hereditary diseases for testing of gene-directed therapy. Research Results in Pharmacology 8(2): 11–26. https://doi.org/10.3897/rrpharmacology.8.82618
Reash NF, James MK, Alfano LN, Mayhew AG, Jacobs M, Iammarino MA, Holsten S, Sakamoto C, Tateishi T, Yajima H, Duong T, de Wolf B, Gee R, Bharucha-Goebel DX, Bravver E, Mori-Yoshimura M, Bushby K, Rufibach LE, Straub V, Lowes LP; Jain COS Consortium (2022) Comparison of strength testing modalities in dysferlinopathy. Muscle 66(2): 159–166. https://doi.org/10.1002/mus.27570 [PubMed]
Shi X, Bai H, Wang J, Wang J, Huang L, He M, Zheng X, Duan Z, Chen D, Zhang J, Chen X, Wang J (2021) Behavioral assessment of sensory, motor, emotion, and cognition in rodent models of intracerebral hemorrhage. Frontiers in Neurology 12: 667511. https://doi.org/10.3389/fneur.2021.667511 [PubMed] [PMC]
Starostina IG, Solovyeva VV, Shevchenko KG, Deev RV, Isaev AA, Rizvanov AA (2012) Formation of the recombinant adenovirus encoding codon-optimized dysferlin gene and analysis of the recombinant protein expression in cell culture in vitro. Genes and Cells [Geny i Kletki] 7: 25–28. https://doi.org/10.23868/gc121567 [in Russian]
Wang D, Liu XY, He QF, Zheng FZ, Chen L, Zheng Y, Zeng MH, Lin YH, Lin X, Chen HZ, Lin MT, Wang N, Wang ZQ, Lin F (2024) Comprehensive proteomic analysis of dysferlinopathy unveiling molecular mechanisms and biomarkers linked to pathological progression. CNS Neuroscience & Therapeutics 30(10): e70065. https://doi.org/10.1111/cns.70065 [PubMed] [PMC]
Yakovlev IA, Emelin AM, Slesarenko YS, Limaev IS, Vetrova IA, Belikova LD, Grafskaia EN, Bobrovsky PA, Pokrovsky MV, Kuzubova EV, Pokrovsky VM, Lebedev PA, Bardakov SN, Isaev AA, Deev R (2023) Dual adeno-associated virus 9 with codon-optimized DYSF gene promotes in vivo muscle regeneration and may decrease inflammatory response in limb girdle muscular dystrophy type R2. International Journal of Molecular Sciences 24(17): 13551. https://doi.org/10.3390/ijms241713551 [PubMed] [PMC]
Zorin VL, Zorina AI, Eremin II, Deev RV, Kopnin PB, Volozhin GA, Pulin AA (2017) Gingiva as a source of stromal cells with high differentiating and reparative potential. Genes and Cells [Geny i Kletki] 12: 37–51. https://doi.org/10.23868/201707014 [in Russian]
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Kuzubova EV, Radchenko AI, Manuylov AA, Korokina AM, Koroleva NV, Yakovlev IA, Isaev AA, Deev RV, Korokin MV

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

