Homozygous mice with mutant protein FUS[1-359] overexpression: Innovative possibilities for ALS treatment
DOI:
https://doi.org/10.18413/rrpharmacology.10.554Abstract
Introduction: This study investigates a mouse model with overexpression of the mutant FUS[1-359] protein, which can be used to evaluate the effectiveness of gene therapy and other pharmacological interventions for amyotrophic lateral sclerosis (ALS). The model enhances the deeper understanding of the mechanisms underlying disease progression and allows for testing new therapeutic strategies.
Materials and Methods: The study utilized tg_hFUS[1-359] animal lines with a transgenic cassette expressing the human mutant FUS[1-359] protein. Animal groups were formed by crossing hemizygous individuals, and analyses were conducted on lifespan, age of disease manifestation, as well as relative copy number and expression levels of the transgenic cassette.
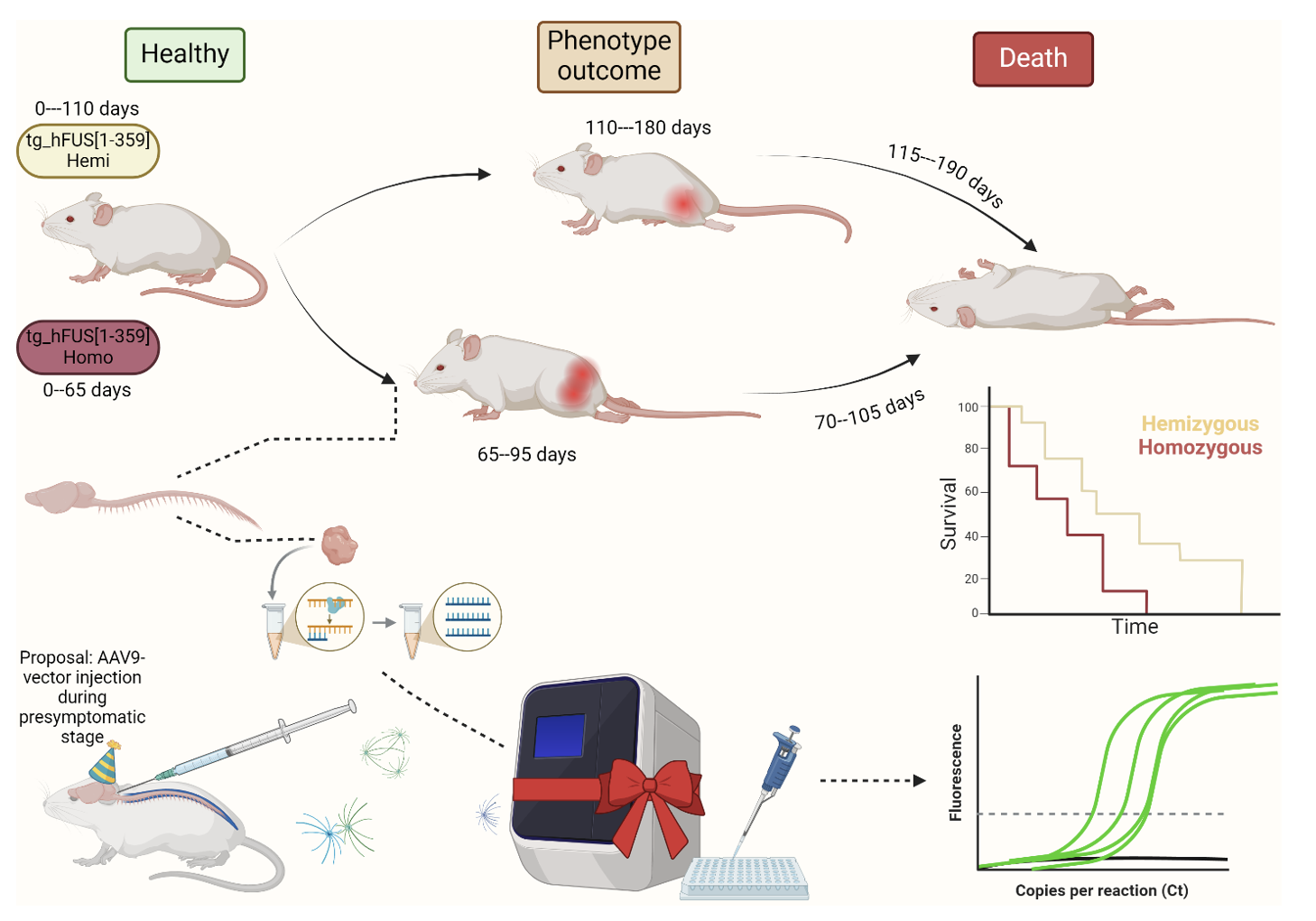
Results and Discussion: The results demonstrated statistically significant differences in the age of onset of initial disease symptoms between homozygous and hemizygous mice. Differences in the copy number of the transgenic insertion were also identified, revealing that homozygous animals exhibited increased expression of the mutant FUS protein in various structures of the central nervous system, consistent with existing literature on ALS pathogenesis.
Conclusion: Mice with hyperexpression of the mutant FUS[1-359] protein represent a promising genetic model for evaluating therapeutic approaches to ALS treatment. This model exhibits clear phenotypic manifestations of the disease and can be utilized for investigating gene therapy methods.
Graphical Abstract
Keywords:
amyotrophic lateral sclerosis, fused in sarcoma, gene expression, gene therapyReferences
Crivello M, Hogg MC, Jirström E, Halang L, Woods I, Rayner M, Coughlan KS, Lewandowski SA, Prehn JHM (2019) Vascular regression precedes motor neuron loss in the FUS (1-359) ALS mouse model. Disease Models & Mechanisms 12(8): dmm040238. https://doi.org/10.1242/dmm.040238 [Pubmed] [PMC]
de Munter J, Babaevskaya D, Wolters EC, Pavlov D, Lysikova E, Kalueff AV, Gorlova A, Oplatchikova M, Pomytkin IA, Proshin A, Umriukhin A, Lesch KP, Strekalova T (2020) Molecular and behavioural abnormalities in the FUS-tg mice mimic frontotemporal lobar degeneration: Effects of old and new anti-inflammatory therapies. Journal of Cellular and Molecular Medicine 24(17): 10251–10257. https://doi.org/10.1111/jcmm.15628 [Pubmed] [PMC]
Funikov SY, Rezvykh AP, Mazin PV, Morozov AV, Maltsev AV, Chicheva MM, Vikhareva EA, Evgen'ev MB, Ustyugov AA (2018) FUS(1-359) transgenic mice as a model of ALS: pathophysiological and molecular aspects of the proteinopathy. Neurogenetics 19(3): 189–204. https://doi.org/10.1007/s10048-018-0553-9 [PubMed]
Grandchamp N (2020) Pharmacodynamic evaluation: gene therapy. In: Hock F, Gralinski M (Eds) Drug Discovery and Evaluation: Methods in Clinical Pharmacology. Springer, Cham, 361–384 pp. https://doi.org/10.1007/978-3-319-68864-0_51
Korokin MV, Gudyrev OS, Pokrovskaya TG, Danilenko LM, Zhernakova NI, Avtina TV, Parshina AV, Pribylov SA, Lebedev PR, Kochkarov AA, Kuzubova EV, Radchenko AI, Koklin IS, Taran EI (2023) Features of bone remodeling and osteoreparation processes in modeling femoral fracture in genetically modified mice with impaired enzymatic regulation of steroid hormone metabolism. Research Results in Pharmacology 9(4): 113–123. https://doi.org/10.18413/rrpharmacology.9.10062
Polikarpova AV, Egorova TV, Bardina MV (2022) Genetically modified animal models of hereditary diseases for testing of gene-directed therapy. Research Results in Pharmacology 8(2): 11–26. https://doi.org/10.3897/rrpharmacology.8.82618
Probert F, Gorlova A, Deikin A, Bettendorff L, Veniaminova E, Nedorubov A, Chaprov KD, Ivanova TA, Anthony DC, Strekalova T (2022) In FUS[1-359]-tg mice O,S-dibenzoyl thiamine reduces muscle atrophy, decreases glycogen synthase kinase 3 beta, and normalizes the metabolome. Biomedicine & Pharmacotherapy 156: 113986. https://doi.org/10.1016/j.biopha.2022.113986 [Pubmed]
Rezvykh A, Shteinberg D, Bronovitsky E, Ustyugov A, Funikov S (2024) Animal models of FUS-proteinopathy: a systematic review. Biochemistry (Moscow) 89(1): 34–56. https://doi.org/10.1134/S0006297924140037 [Pubmed]
Shelkovnikova TA, Peters OM, Deykin AV, Connor-Robson N, Robinson H, Ustyugov AA, Bachurin SO, Ermolkevich TG, Goldman IL, Sadchikova ER, Kovrazhkina EA, Skvortsova VI, Ling SC, Da Cruz S, Parone PA, Buchman VL, Ninkina NN (2013) Fused in sarcoma (FUS) protein lacking nuclear localization signal (NLS) and major RNA binding motifs triggers proteinopathy and severe motor phenotype in transgenic mice. The Journal of Biological Chemistry 288(35): 25266–25274. https://doi.org/10.1074/jbc.m113.492017 [Pubmed] [PMC]
Yazdani A, Alirezaie Z, Motamedi MJ, Amani J (2018) Gene therapy: a new approach in modern medicine. International Journal of Medical Research 5(3): 105–107. https://doi.org/10.29252/IJMR-050304
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Zhunusov NS

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

