Investigation of imidazole-4,5-dicarboxylic acid derivatives activity on visceral pain in mice
DOI:
https://doi.org/10.18413/rrpharmacology.11.773Abstract
Introduction: Glutamate plays an important role in the modulation of nociception. Experimental studies on rodents have shown that mGluR1 receptor inhibitors demonstrate antinociceptive potential in acute pain models. New ligands of the glutamate NMDA-receptor complex are imidazole-4,5-dicarboxylic acid derivatives, which conformational rigidity allows increasing selectivity interaction and decreasing a number of side effects with the implementiation a high analgesic potential.
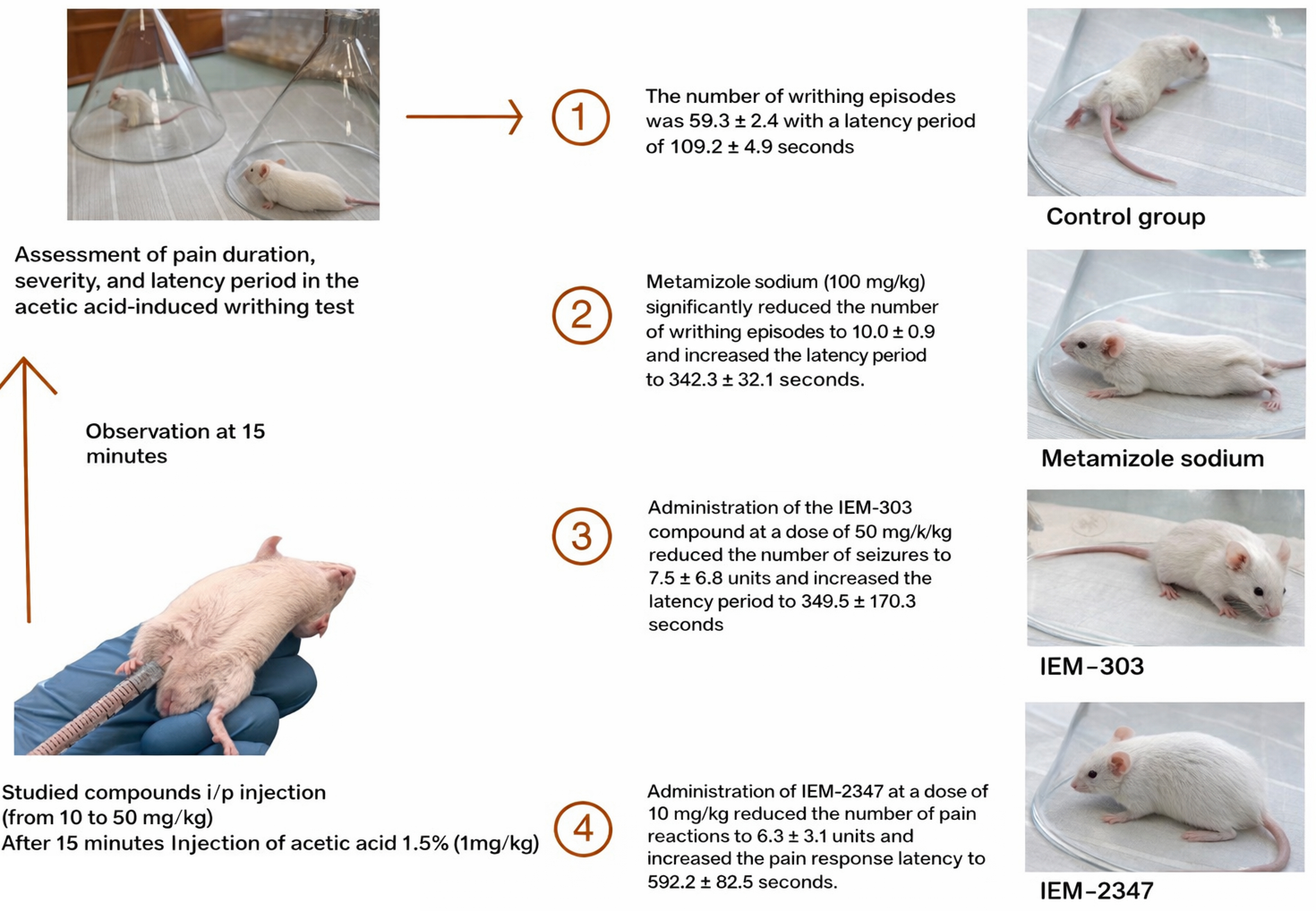
Materials and Methods: Intraperitoneal administration of algogen – 1.5% acetic acid solution –causes chemical pain irritation, manifested by specific animal movements called writhing. Fifteen minutes before the administration of 1.5% acetic acid solution, mice were intraperitoneally administered the experimental substances: IEM-303 at a dose of 50 mg/kg and IEM-2347 at doses of 10, 20, and 40 mg/kg. The control group of animals received a physiological solution in an equivalent volume, and the comparison group received metamizole sodium at a dose of 100 mg/kg. The analgesic effect was assessed by the ability of the drugs to reduce the number of writhings in comparison with the control group.
Results: The introduction of substance IEM-2347 at a concentration of 10 mg/kg led to a decrease in the number of writhings to 6.3 ± 3.1 units (by 89.4%), which significantly exceeds the indicators obtained in the group receiving metamizole sodium. With a further increase in IEM-2347 concentration to 20-40 mg/kg, complete suppression of the pain response was observed in 100% of animals.
Discussion: It can be assumed that the elongation of radicals in the benzene ring at nitrogen atoms has an effect enhancing the analgesic potential of the imidazole dicarboxylic acid derivative.
Conclusion: High analgesic potential with toxicological safety of imidazole-4,5-dicarboxylic acid derivatives allows them to be considered as promising pain relievers.
Graphical Abstract
Keywords:
glutamate, NMDA-receptor antagonists, imidazole-4,5-dicarboxylic acid derivatives, analgesic effect, visceral pain, miceReferences
Alyautdin RN (2019) Pharmacology. GEOTAR-Media, Moscow, 352 pp. [in Russian]
Ahn DK, Kim KH, Jung CY, Choi HS, Lim EJ, Youn DH, Bae YC (2005) Role of peripheral group I and II metabotropic glutamate receptors in IL-1beta-induced mechanical allodynia in the orofacial area of conscious rats. Pain 118: 53–60. https://doi.org/10.1016/j.pain.2005.07.017[PubMed]
Barinov AN (2010) The role of homosynaptic stimulus-dependent neuronal plasticity (the wind-up phenomenon) in the chronification of pain syndromes. Consilium Medicum (Neurology and Rheumatology) 12(2): 53–59. [in Russian]
Bespalov AYu, Zvartau EE (2000) Neuropsychopharmacology of NMDA-receptor antagonists. Nevsky Dialect, St. Petersburg, 297pр. [in Russian]
Camilleri M, Coulie B, Tack JF (2001) Visceral hypersensitivity: facts, speculations and challenges. Gut 48(1): 125–131. https://doi.org/10.1136/gut.48.1.125 [PubMed] [PMC]
Cervero F (2000) Visceral pain-central sensitization. Gut 47(Suppl. 4): iv56–iv57. https://doi.org/10.1136/gut.47.suppl_4.iv56 [PubMed] [PMC]
Davydova ON, Boldyrev AA (2007) Glutamate receptors in cells of the nervous and immune systems. Annals of Clinical and Experimental Neurology 1(7): 26-43. [in Russian]
Dogrul A, Ossipov MH, Lai J, Malan TP Jr, Porreca F (2000) Peripheral and spinal antihyperalgesic activity of SIB-1757, a metabotropic glutamate receptor (mGLUR(5)) antagonist, in experimental neuropathic pain in rats. Neuroscience Letters 292(2): 115–118. https://doi.org/10.1016/s0304-3940(00)01458-0 [PubMed]
Farmer AD, Aziz Q (2009) Visceral pain hypersensitivity in functional gastrointestinal disorders. British Medical Bulletin 91: 123–126. https://doi.org/10.1093/bmb/ldp026 [PubMed]
Hao S, Lin S, Tao W, Zhuo M (2025) Cortical potentiation in chronic neuropathic pain and the future treatment. Pharmaceuticals 18(3): 363. https://doi.org/10.3390/ph18030363[PubMed] [PMC]
Kukushkin ML, Tabeeva GR, Podchufarova EV (2011) Pain syndrome: pathophysiology, clinical features, treatment. IMAPress:Moscow, 72 pp. [in Russian]
Mazzitelli M, Presto P, Antenucci1 N, Meltan1S, Neugebauer V (2022) Recent advances in the modulation of pain by the metabotropic glutamate receptors. Cells 11(16): 2608.https://doi.org/10.3390/cells11162608 [PubMed] [PMC]
Mironov AN, Bunjatjan ND (2013) Guidelines for conducting preclinical research of medicines. Vol. 1. Grif and K, Moscow, 944 pp. [in Russian]
Ovsyannikov VG, Shlyk SV, Boychenko AE, Alekseev VV, Alekseeva NS, Kaplunova OA (2013) Features of the pathogenesis of visceral pain. Medical Bulletin of the South of Russia [Meditsinskiy Vestnik Yuga Rossii] 3: 12–19. [in Russian]
Urch CE, Walsh T, Caraceni A, Fainsinger R. et al. (2009) Pathophysiology of cancer pain. Palliative medicine. Expert Consult: Online and print Saunders 244: 1378–1384.
Walker K, Bowes M, Panesar M, Davis A, Gentry C, Kesingland A, Gasparini F, Spooren W, Stoehr N, Pagano A, Flor PJ, Vranesic I, Lingenhoehl K, Johnson EC, Varney M, Urban L, Kuhn R (2001) Metabotropic glutamate receptor subtype 5 (mGlu5) and nociceptive function. Selective blockade of mGlu5 receptors in models of acute, persistent and chronic pain. Neuropharmacology 40(1): 1–9. https://doi.org/10.1016/s0028-3908(00)00113-1 [PubMed]
Yakovleva EE, Kamalova MT, Brusina MA, Bychkov ER, Piotrovskiy LB, Shabanov PD (2024) Analgesic activity of new ligands of the NMDA receptor complex. Reviews on Clinical Pharmacology and Drug Therapy [Obzory po Klinicheskoj Farmakologii i Lekarstvennoj Terapii] 22(2): 171–178. https://doi.org/10.17816/RCF624859 [in Russian]
Yaksh TL, et al. (1999) The spinal biology in humans and animals of pain states generated by persistent small afferent input. Proceedings of the National Academy of Sciences 96(14): 7680–7686. https://doi.org/10.1073/pnas.96.14.7680 [PubMed] [PMC]
Zhu CZ, Hsieh G, Ei-Kouhen O, Wilson SG, Mikusa JP, Hollingsworth PR, Chang R, Moreland RB, Brioni J, Decker MW, Honore P (2005) Role of central and peripheral mGluR5 receptors in post-operative pain in rats. Pain 114(1-2): 195–202. https://doi.org/10.1016/j.pain.2004.12.016 [PubMed]
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Yakovleva EE, Kamalova MT, Yakovleva VG, Bychkov ER, Shabanov PD

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

