Cytotoxicity study of transdermal desloratadine delivery system on murine melanoma and human dermal fibroblast cell cultures
DOI:
https://doi.org/10.18413/rrpharmacology.11.731Аннотация
treat and prevent allergic conditions, reducing the adverse effects associated with oral administration.
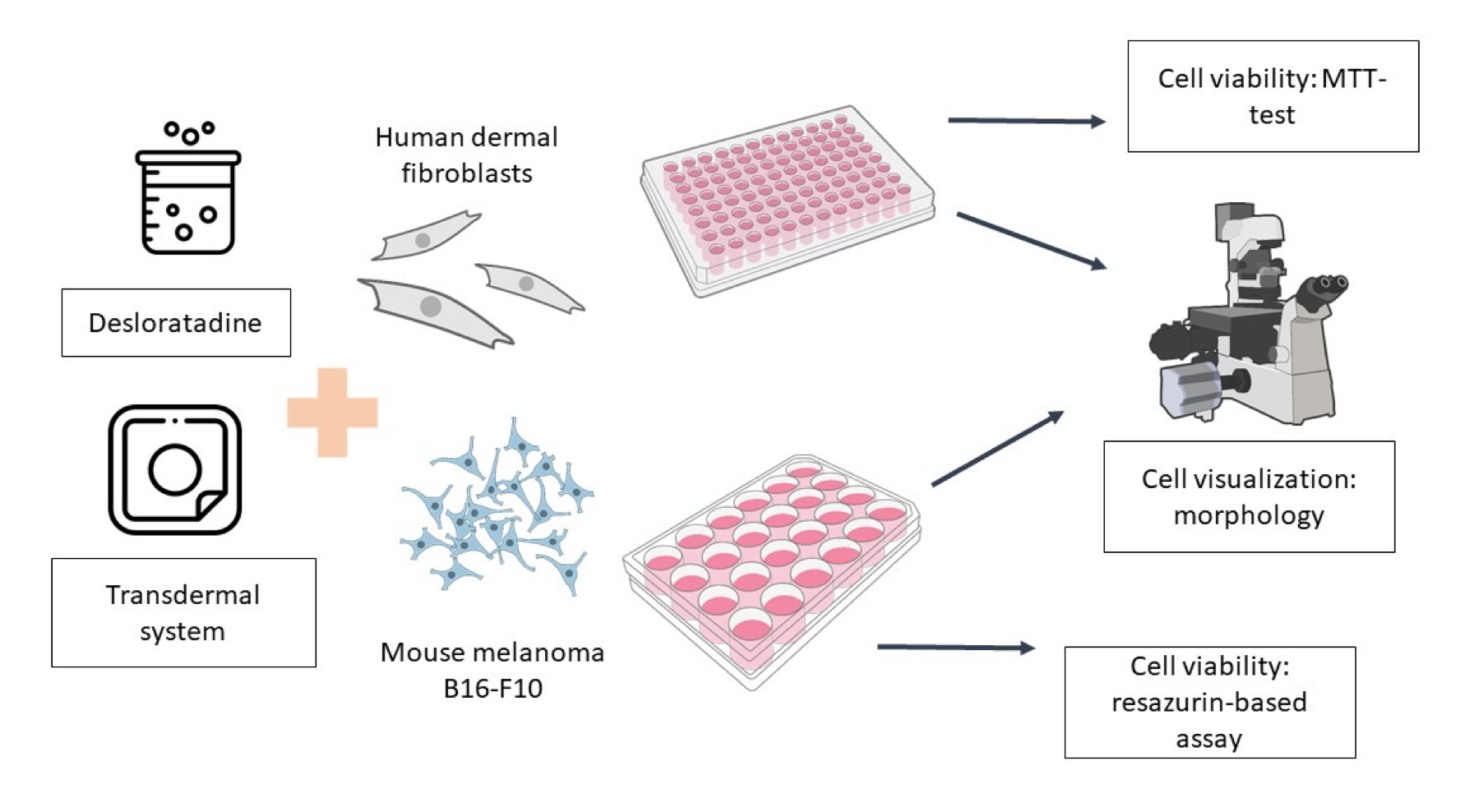
Materials and Methods: We evaluated the cytotoxicity of the transdermal desloratadinedelivery system in micellar and ionic forms on murine melanoma and human dermal fibroblast cell cultures using the MTT assay and resazurin live cell assay.
Results: The results of the MTT assay demonstrated that desloratadine in micellar and ionic forms had a more pronounced cytotoxic effect on murine melanoma cells than on human dermal fibroblasts. The transdermal system had no effect on cell viability. Desloratadine in micellar or ionic form reduced cell viability: the survival rate of murine melanoma cells was below 50%, while when incubated with the transdermal system, the viability of human dermal fibroblasts was above 70%, indicating no toxicity.
Discussion: The polyvinylpyrrolidone-based transdermal system is not cytotoxic, but the active ingredient, desloratadine, features antiproliferative activity, to a greater extent, in relation to tumor cells.
Conclusion: The obtained results demonstrated the cytotoxic effect of micellar and ionic desloratadine on the tumor culture of murine melanoma and the biocompatibility of the transdermal desloratadine delivery system with human dermal fibroblasts.
Графическая аннотация
Ключевые слова:
antihistamine, biocompatibility, human dermal fibroblasts, murine melanoma, patch, cytotoxicityБиблиографические ссылки
Ageev AP, Zaborovskiy AV, Yunina DV, Tararina LA, Devkota MK, Andreev DN, Pyanzina AE, Shlyapkina VI, Buldygina YA, Kulikov OA, Pyataev NA (2024) Development of a transcutaneous form of desloratadine. Research Results in Pharmacology 10(2): 57–64. https://doi.org/10.18413/rrpharmacology.10.487
Berger WE, Schenkel EJ, Mansfield LE, Desloratadine Study Group (2002) Safety and efficacy of desloratadine 5 mg in asthma patients with seasonal allergic rhinitis and nasal congestion. Annals of Allergy, Asthma, and Immunology 89(5): 485–491. https://doi.org/10.1016/S1081-1206(10)62086-8
Danciu C, Falamas A, Dehelean C, Soica C, Radeke H, Barbu-Tudoran L, Bojin F, Pînzaru SC, Munteanu MF (2013) A characterization of four B16 murine melanoma cell sublines molecular fingerprint and proliferation behavior. Cancer Cell International 13: 75. https://doi.org/10.1186/1475-2867-13-75
Lotfi A, Li H, Dao DV, Prusty G (2019) Natural fiber-reinforced composites: A review on material, manufacturing, and machinability. Journal of Thermoplastic Composite Materials 34(3): 089270571984454. https://doi.org/10.1177/0892705719844546
Sadowska-Woda I, Sychta B, Rachel M, Bieszczad-Bedrejczuk E (2010) Protective effect of desloratadine against oxidative stress in human erythrocytes in vitro. Environmental Toxicology and Pharmacology 30(2): 141–146. https://doi.org/1016/j.etap.2010.05.001 [PubMed]
Kawahara K, Tojo K (2007) Skin Irritation in Transdermal Drug Delivery Systems: A Strategy for its Reduction. Pharmaceutical Research 24: 399–408. https://doi.org/10.1007/s11095-006-9165-4
Загрузки
Опубликован
Как цитировать
Выпуск
Раздел
Лицензия
Copyright (c) 2025 Brodovskaya EP, Al-Hajj Ayub AM, Semikov DO, Ageev VP, Zaborovskiy AV, Tararina LA, Yunina DV, Litvinov SA, Dadaeva IM, Kheladze NV, Andreev DN, Lobanova EG, Pyataev NA (2025) Cytotoxicity study of transdermal desloratadine delivery system on muri

Это произведение доступно по лицензии Creative Commons «Attribution» («Атрибуция») 4.0 Всемирная.
 Русский
Русский
 English
English

