Epoetin-alfa-induced osteogenesis in the bone organoid model
DOI:
https://doi.org/10.18413/rrpharmacology.10.517Abstract
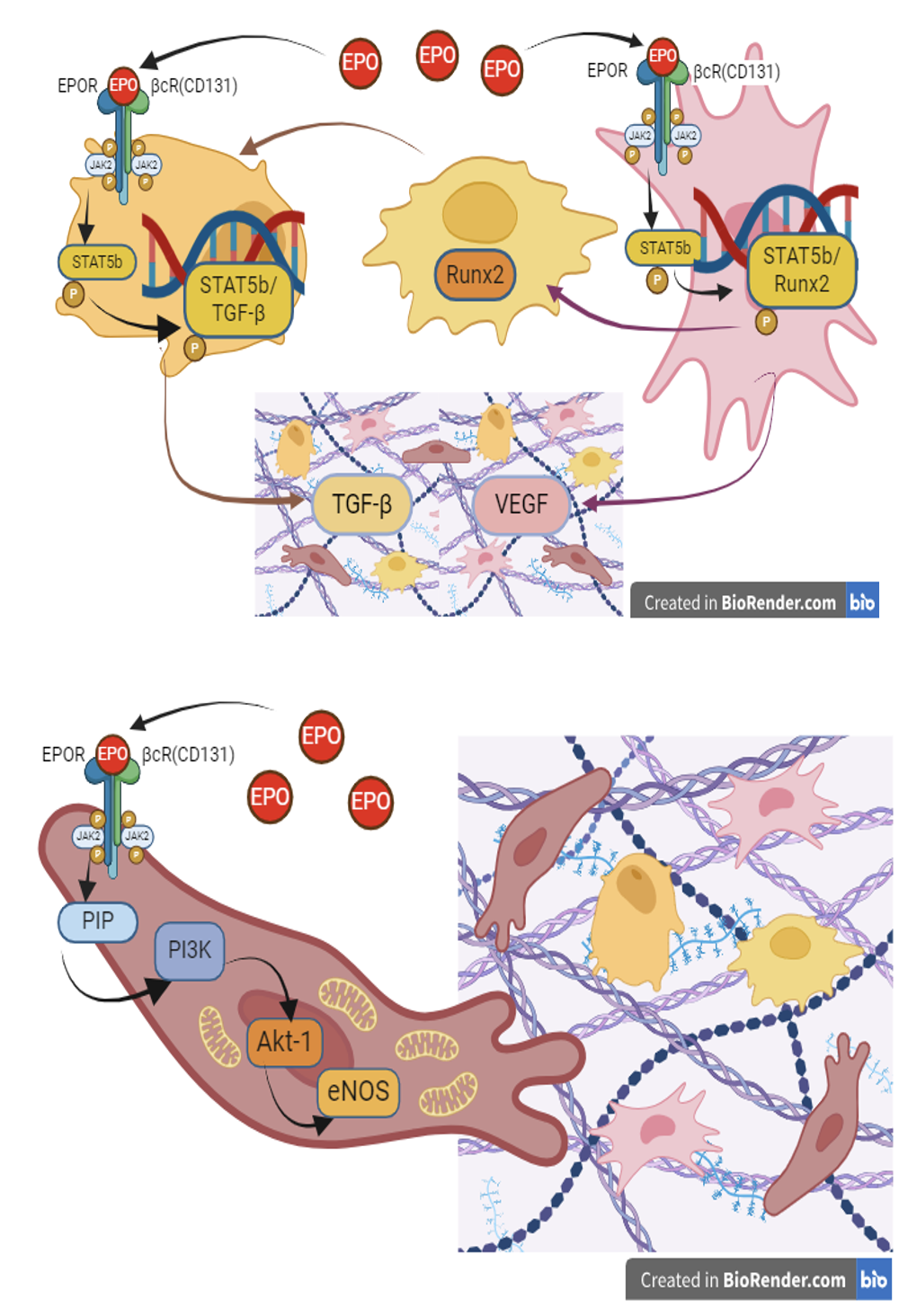
Introduction: Erythropoietin interacts ETwith EPOR/βcR(CD131) and activates the non-canonical pathway JAK/STAT and PI3K/AKT. Phosphorylated STAT subunits were found to get dimerized and translocated into the nucleus to activate the expression of the Runx-2 and TGF-β genes. The purpose of the study was to evaluate the effectiveness of osteogenesis when using epoetin alpha as an osteoinducer in an experimental model of a bone organoid.
Materials and Methods: The study was conducted on human mesenchymal stem cells (hMSC) and trabecular bone organoids (TBO). Photometry and microscopy were used to assess the effects of drugs on proliferation and differentiation of hMSС and the formation of osteogenic tissue in TBO. The volume of connective tissue structures and the activity of mitochondria was determined in cells that form fibrovascular osteogenic tissue in TBO.
Results: Epoetin alpha was found to have a dose-dependent osteoinductive effect in TBO. At a dose of 200 IU/mL, epoetin alpha has an inhibitory effect, whereas at a dose of 20 IU/mL, it activates the proliferation and differentiation of hMSC in the osteogenic direction by inducing osteogenesis in TBO.
Conclusion: Epoetin alpha has a protective effect on osteogenesis in TBO inducing the proliferation of osteoblasts and endotheliocytes, stimulating the growth of connective tissue structures of the bone organoid with the formation of osteogenic tissue along with increasing the activity of mitochondria and reducing intracellular generation of reactive oxygen species.
Graphical Abstract
Keywords:
epoetin alfa, trabecular bone organoid, osteogenesisReferences
Belyaeva VS, Stepenko YV, Lyubimov II, Kulikov AL, Tietze AA, Kochkarova IS, Martynova OV, Pokopeyko ON, Krupen’kina LA, Nagikh AS, Pokrovskiy VM, Patrakhanov EA, Belashova AV, Lebedev PR, Gureeva AV (2020) Non-hematopoietic erythropoietin-derived peptides for atheroprotection and treatment of cardiovascular diseases. Research Results in Pharmacology 6(3): 75–86. https://doi.org/10.3897/rrpharmacology.6.58891
Berg T, Doppelt-Flikshtain O, Coyac BR, Zigdon-Giladi H (2023) Oral fibroblasts rescue osteogenic differentiation of mesenchymal stem cells after exposure to Zoledronic acid in a paracrine effect. Frontiers in Pharmacology 14: 1172705. https://doi.org/10.3389/fphar.2023.1172705 [PubMed] [PMC]
Bruderer M, Richards RG, Alini M, Stoddart MJ (2014) Role and regulation of RUNX2 in osteogenesis. European Cells & Materials 28: 269–286. https://doi.org/10.22203/ecm.v028a19 [PubMed]
Broxmeyer HE (2013) Erythropoietin: multiple targets, actions, and modifying influences for biological and clinical consideration. Journal of Experimental Medicine 210(2): 205–208. https://doi.org/10.1084/jem.20122760 [PubMed]
Dai X, Liu L, Ouyang J, Li X, Zhang X, Lan Q, Xu T (2017) Coaxial 3D bioprinting of self-assembled multicellular heterogeneous tumor fibers. Scientific Reports 7(1): 1457. https://doi.org/10.1038/s41598-017-01581-y [PubMed] [PMC]
Dieudonne FX, Sévère N, Biosse-Duplan M, Weng JJ, Su Y, Marie PJ (2013) Promotion of osteoblast differentiation in mesenchymal cells through Cbl-mediated control of STAT5 activity. Stem Cells 31(7): 1340–1349. https://doi.org/10.1002/stem.1380 [PubMed]
Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM (1999) Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399(6736): 601–605. https://doi.org/10.1038/21224[PubMed]
Gambini E, Martinelli I, Stadiotti I, Vinci MC, Scopece A, Eramo L, Sommariva E, Resta J, Benaouadi S, Cogliati E, Paolin A, Parini A, Pompilio G, Savagner F (2020) Differences in mitochondrial membrane potential identify distinct populations of human cardiac mesenchymal progenitor cells. International Journal of Molecular Sciences 21(20): 7467. https://doi.org/10.3390/ijms21207467 [PubMed]
Garcia P, Speidel V, Scheuer C, Laschke MW, Holstein JH, Histing T, Pohlemann T, Menger MD (2011) Low dose erythropoietin stimulates bone healing in mice. Journal of Orthopaedic Research 29(2): 165–172. https://doi.org/10.1002/jor.21219 [PubMed]
Gazit D, Karmish M, Holzman L, Bab I (1990) Regenerating marrow induces systemic increase in osteo- and chondrogenesis. Endocrinology 126(5): 2607–2613. https://doi.org/10.1210/endo-126-5-2607 [PubMed]
GBD 2021 Other Musculoskeletal Disorders Collaborators (2023) Global, regional, and national burden of other musculoskeletal disorders, 1990-2020, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatology 5(11): e670–e682. https://doi.org/10.1016/S2665-9913(23)00232-1 [PubMed] [PMC]
Fisslthaler B, Benzing T, Busse R, Fleming I (2003) Insulin enhances the expression of the endothelial nitric oxide synthase in native endothelial cells: a dual role for Akt and AP-1. Nitric Oxide 8(4): 253–261. https://doi.org/10.1016/s1089-8603(03)00042-9 [PubMed]
Hofmann E, Eggers B, Heim N, Kramer FJ, Nokhbehsaim M, Götz W (2022) Bevacizumab and sunitinib mediate osteogenic and pro-inflammatory molecular changes in primary human alveolar osteoblasts in vitro. Odontology 110(4): 634–647. https://doi.org/10.1007/s10266-022-00691-y [PubMed]
Holstein JH, Menger MD, Scheuer C, Meier C, Culemann U, Wirbel RJ, Garcia P, Pohlemann T (2007) Erythropoietin (EPO): EPO-receptor signaling improves early endochondral ossification and mechanical strength in fracture healing. Life Sciences 80(10): 893–900. https://doi.org/10.1016/j.lfs.2006.11.023 [PubMed]
Horvath P, Aulner N, Bickle M, Davies AM, Nery ED, Ebner D, Montoya MC, Östling P, Pietiäinen V, Price LS, Shorte SL, Turcatti G, von Schantz C, Carragher NO (2016) Screening out irrelevant cell-based models of disease. Nature Reviews Drug Discovery 15(11): 751–769. https://doi.org/10.1038/nrd.2016.175 [PubMed]
Jelkmann W (2004) Molecular biology of erythropoietin. Internal Medicine 43(8): 649–59. https://doi.org/10.2169/internalmedicine.43.649 [PubMed]
Jérôme V, Freitag R, Schüler D, Mickoleit F (2019) SEAP activity measurement in reporter cell-based assays using BCIP / NBT as substrate. Analytical Biochemistry 585: 113402. https://doi.org/10.1016/j.ab.2019.113402 [PubMed]
Khan S, Villalobos MA, Choron RL, Chang S, Brown SA, Carpenter JP, Tulenko TN, Zhang P (2017) Fibroblast growth factor and vascular endothelial growth factor play a critical role in endotheliogenesis from human adipose-derived stem cells. Journal of Vascular Surgery 65(5): 1483–1492. https://doi.org/10.1016/j.jvs.2016.04.034 [PubMed]
Kuksal N, Chalker J, Mailloux RJ (2017) Progress in understanding the molecular oxygen paradox - function of mitochondrial reactive oxygen species in cell signaling. Biological Chemistry 398(11): 1209–1227. https://doi.org/10.1515/hsz-2017-0160 [PubMed]
Nairz M, Sonnweber T, Schroll A, Theurl I, Weiss G (2012) The pleiotropic effects of erythropoietin in infection and inflammation. Microbes and Infection 14(3): 238–246. https://doi.org/10.1016/j.micinf.2011.10.005 [PubMed]
Pittenger MF (2008) Mesenchymal stem cells from adult bone marrow. Methods in Molecular Biology 449: 27–44. https://doi.org/10.1007/978-1-60327-169-1_2
Pusztaszeri MP, Seelentag W, Bosman FT (2006) Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. Journal of Histochemistry & Cytochemistry 54(4): 385–395. https://doi.org/10.1369/jhc.4A6514.2005 [PubMed]
Qin XG, Hua Z, Shuang W, Wang YH, Cui YD (2010) Effects of matrine on HepG2 cell proliferation and expression of tumor relevant proteins in vitro. Pharmaceutical Biology 48(3): 275–281. https://doi.org/10.3109/13880200903104101 [PubMed]
Rakhit RD, Mojet MH, Marber MS, Duchen MR (2001) Mitochondria as targets for nitric oxide-induced protection during simulated ischemia and reoxygenation in isolated neonatal cardiomyocytes. Circulation 103(21): 2617–2623. https://doi.org/10.1161/01.cir.103.21.2617 [PubMed]
Schindeler A, McDonald MM, Bokko P, Little DG (2008) Bone remodeling during fracture repair: The cellular picture. Seminars in Cell & Developmental Biology 19(5): 459–466. https://doi.org/10.1016/j.semcdb.2008.07.004[PubMed]
Stepenko YV, Shmigerova VS, Kostina DA, Shcheblykina OV, Zhernakova NI, Solin AV, Koroleva NV, Markovskaya VA, Dudnikova OV, Bolgov AA (2024) Study of the neuroprotective properties of the heteroreceptor EPOR/CD131 agonist of peptide structure in tau-proteinopathy modeling. Research Results in Pharmacology 10(2): 41–47. https://doi.org/10.18413/rrpharmacology.10.492
Tsiftsoglou AS (2021) Erythropoietin (EPO) as a Key Regulator of Erythropoiesis, Bone Remodeling and Endothelial Transdifferentiation of Multipotent Mesenchymal Stem Cells (MSCs): Implications in Regenerative Medicine. Cells 10(8): 2140. https://doi.org/10.3390/cells10082140 [PubMed] [PMC]
Uggeri J, Gatti R, Belletti S, Scandroglio R, Corradini R, Rotoli BM, Orlandini G (2004) Calcein-AM is a detector of intracellular oxidative activity. Histochemistry and Cell Biology 122(5): 499–505. https://doi.org/10.1007/s00418-004-0712-y [PubMed]
Yin T, Li L. (2006) The stem cell niches in bone. Journal of Clinical Investigation 116(5): 1195–1201. https://doi.org/10.1172/JCI28568 [PubMed]
Zhao D, Saiding Q, Li Y, Tang Y, Cui W (2024) Bone Organoids: Recent advances and future challenges. Advanced Healthcare Materials. 13(5): e2302088. https://doi.org/10.1002/adhm.202302088
Zubareva EV, Nadezhdin SV, Burda YE, Nadezhdina NA, Gashevskaya AS (2019) Pleiotropic effects of Erythropoietin. Influence of Erythropoietin on processes of mesenchymal stem cells differentiation. Research Results in Pharmacology 5(1): 53–66. https://doi.org/10.3897/rrpharmacology.5.33457
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Nadezhdina NA, Gudyrev OS, Nadezhdin SV, Danilenko LM, Dolzhikov AA, Kremleva KK, Pokrovskaya TG

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

