The effect of acute swimming stress, corticosterone, dexamethasone, and fludrocortisone on anxiety-like behavior in mice
DOI:
https://doi.org/10.18413/rrpharmacology.11.725Abstract
Introduction: Acute swimming stress (ASS) exerts a biphasic effect on anxiety-like behavior (ALB) in mice, inducing an enhancement and a subsequent decrease in ALB 1 h and 24 h after exposure, respectively. Presumably, this effect may be caused by the activation of mineralocorticoid and glucocorticoid receptors, both during the immediate response to acute stress and after its termination at the phase of adaptive changes. Aim of the Research: Comparative study of the effects of acute swimming stress, corticosterone, dexamethasone, and fludrocortisone on ALB in mice 1 h and 24 h after stress exposure or administration of the studied substances.
Materials and Methods: To model ASS in adult male ICR mice, the forced swimming test (FST) was employed. ALB was assessed in mice in the open field test 1 h and 24 h after FST or systemic administration of corticosterone (20 mg/kg), dexamethasone (10 mg/kg), and fludrocortisone (0.04 mg/kg).
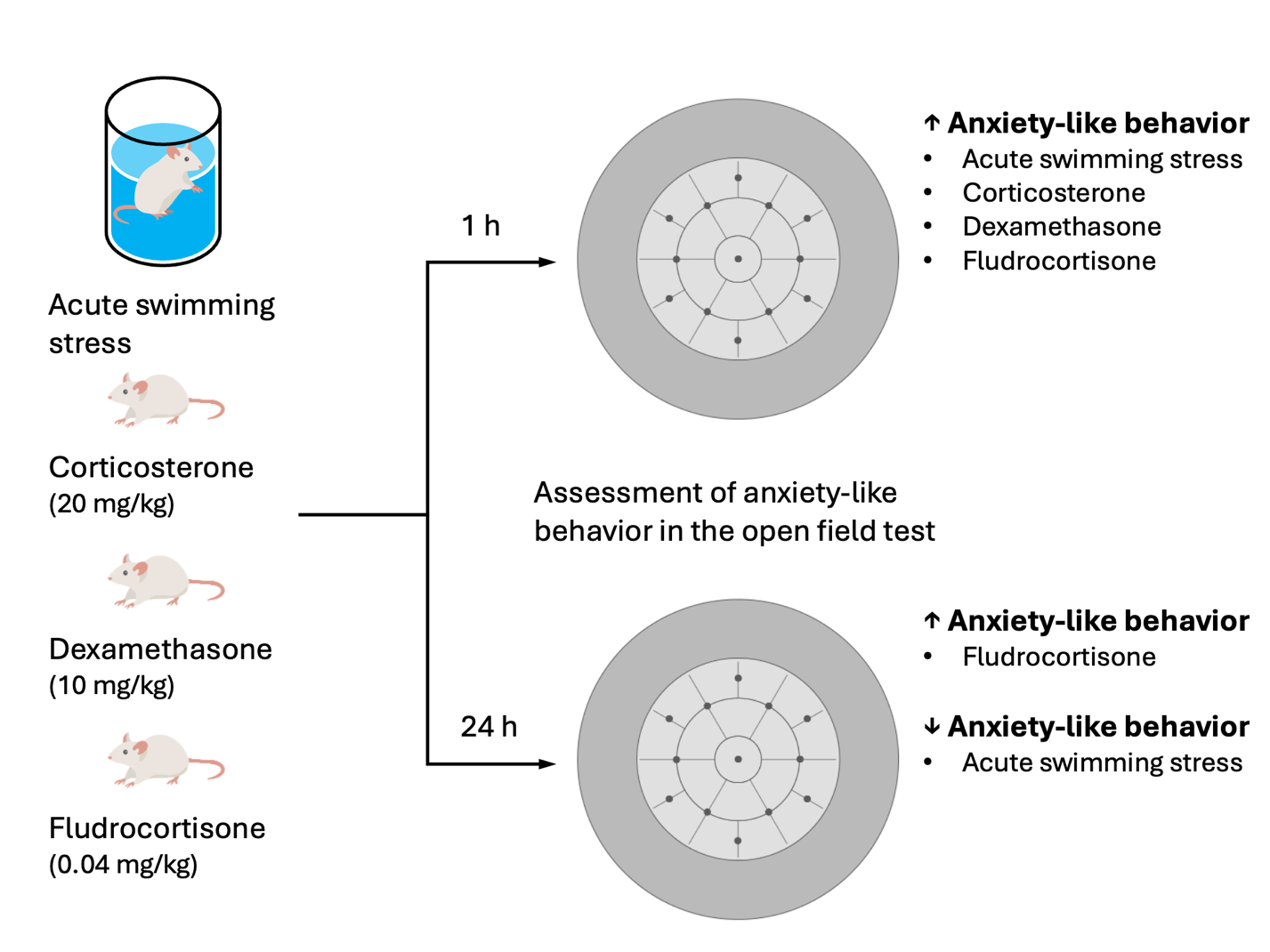
Results: Central activity and anxiety index were increased 1 h after exposure to FST, while an increase in the anxiety index was observed 24 h after exposure to FST in the open field test compared to the non-stressed mice. Corticosterone, dexamethasone, and fludrocortisonedecreased central activity and anxiety index 1 h after the administration, compared to the control group. However, fludrocortisone decreased central activity and total locomotor activity 24 h after administration compared to the control group.
Conclusion: A similar pattern of enhancement of anxiety-like behavior was observed in mice 1 h after FST or the administration of corticosterone (single dose 20 mg/kg, i.p.), dexamethasone(single dose 10 mg/kg, i.p.), and fludrocortisone (single dose 0.04 mg/kg, i.p.). Nonetheless, 24 h after exposure to stress or administration of the studied substances, FST decreased ALB, fludrocortisone enhanced ALB, while corticosterone and dexamethasone showed no effect on ALB in mice 24 h after exposure to stress or administration of the studied substances.
Graphical Abstract
Keywords:
acute swimming stress, anxiety, corticosterone, dexamethasone, fludrocortisone, mouse, open field testReferences
Atrooz F, Alkadhi KA, Salim S (2021) Understanding stress: Insights from rodent models. Current Research in Neurobiology 2: 100013. https://doi.org/10.1016/j.crneur.2021.100013 [PubMed] [PMC]
Avital A, Richter-Levin G, Leschiner S, Spanier I, Veenman L, Weizman A, Gavish M (2001) Acute and repeated swim stress effects on peripheral benzodiazepine receptors in the rat hippocampus, adrenal, and kidney. Neuropsychopharmacology 25(5): 669–678. https://doi.org/10.1016/S0893-133X(01)00286-X [PubMed]
Bertholomey ML, Nagarajan V, Smith DM, Torregrossa MM (2022) Sex- and age-dependent effects of chronic corticosterone exposure on depressive-like, anxiety-like, and fear-related behavior: Role of amygdala glutamate receptors in the rat. Frontiers in Behavioral Neuroscience 16: 950000. https://doi.org/10.3389/fnbeh.2022.950000 [PubMed] [PMC]
Browne CA, Hanke J, Rose C, Walsh I, Foley T, Clarke G, Schwegler H, Cryan JF, Yilmazer-Hanke D (2014) Effect of acute swim stress on plasma corticosterone and brain monoamine levels in bidirectionally selected DxH recombinant inbred mouse strains differing in fear recall and extinction. Stress 17(6): 471–483. https://doi.org/10.3109/10253890.2014.954104 [PubMed] [PMC]
Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD (2012) The mouse forced swim test. Journal of Visualized Experiments 59: e3638. https://doi.org/10.3791/3638[PubMed] [PMC]
Christianson JP, Drugan RC, Flyer JG, Watkins LR, Maier SF (2013) Anxiogenic effects of brief swim stress are sensitive to stress history. Progress in Neuro-Psychopharmacology & Biological Psychiatry 44: 17–22. https://doi.org/10.1016/j.pnpbp.2013.01.011 [PubMed] [PMC]
Commons KG, Cholanians AB, Babb JA, Ehlinger DG (2017) The rodent forced swim test measures stress-coping strategy, not depression-like behavior. ACS Chemical Neuroscience 8(5): 955–960. https://doi.org/10.1021/acschemneuro.7b00042 [PubMed] [PMC]
Dieterich A, Srivastava P, Sharif A, Stech K, Floeder J, Yohn SE, Samuels BA (2019) Chronic corticosterone administration induces negative valence and impairs positive valence behaviors in mice. Translational Psychiatry 9(1): 337. https://doi.org/10.1038/s41398-019-0674-4 [PubMed] [PMC]
Dinel AL, Guinobert I, Lucas C, Blondeau C, Bardot V, Ripoche I, Berthomier L, Pallet V, Layé S, Joffre C (2019) Reduction of acute mild stress corticosterone response and changes in stress-responsive gene expression in male Balb/c mice after repeated administration of a Rhodiola rosea L. root extract. Food Science & Nutrition 7(11): 3827–3841. https://doi.org/10.1002/fsn3.1249 [PubMed] [PMC]
Fediuc S, Campbell JE, Riddell MC. (2006) Effect of voluntary wheel running on circadian corticosterone release and on HPA axis responsiveness to restraint stress in Sprague-Dawley rats. Journal of Applied Physiology 100(6): 1867–1875. https://doi.org/10.1152/japplphysiol.01416.2005 [PubMed]
Hartmann J, Bajaj T, Klengel C, Chatzinakos C, Ebert T, Dedic N, McCullough KM, Lardenoije R, Joëls M, Meijer OC, McCann KE, Dudek SM, Sarabdjitsingh RA, Daskalakis NP, Klengel T, Gassen NC, Schmidt MV, Ressler KJ (2021) Mineralocorticoid receptors dampen glucocorticoid receptor sensitivity to stress via regulation of FKBP5. Cell Reports 35(9): 109185. https://doi.org/10.1016/j.celrep.2021.109185 [PubMed] [PMC]
Hoeijmakers L, Harbich D, Schmid B, Lucassen PJ, Wagner KV, Schmidt MV, Hartmann J (2014) Depletion of FKBP51 in female mice shapes HPA axis activity. PLoS One 9(4): e95796. https://doi.org/10.1371/journal.pone.0095796 [PubMed] [PMC]
Kloet de ER, Molendijk ML (2016) Coping with the forced swim stressor: Towards understanding an adaptive mechanism. Neural Plasticity 2016: 6503162. https://doi.org/10.1155/2016/6503162 [PubMed] [PMC]
Kloet de ER (2022) Brain mineralocorticoid and glucocorticoid receptor balance in neuroendocrine regulation and stress-related psychiatric etiopathologies. Current Opinion in Endocrine and Metabolic Research 24: 100352. https://doi.org/10.1016/j.coemr.2022.100352 [PubMed] [PMC]
Kudryashov NV, Naplekova PL, Volkova AV, Kasabov KA, Narkevich VB, Kudrin VS, Kalinina TS, Voronina TA (2022) Effects of acute swimming stress on the behavioral and neurochemical effects of pyrazolo[c]pyridine derivative GIZh-72 and diazepam in BALB/c and C57BL/6 mice. Neuroscience and Behavioral Physiology 52: 135–149. https://doi.org/1007/s11055-022-01215-5
Laviolle B, Nesseler N, Massart C, Bellissant E (2014) Fludrocortisone and hydrocortisone, alone or in combination, on in vivo hemodynamics and in vitro vascular reactivity in normal and endotoxemic rats: a randomized factorial design study. Journal of Cardiovascular Pharmacology 63(6): 488–496. https://doi.org/10.1097/FJC.0000000000000072 [PubMed]
Molendijk ML, Kloet de ER (2022) Forced swim stressor: Trends in usage and mechanistic consideration. The European Journal of Neuroscience 55(9-10): 2813–2831. https://doi.org/10.1111/ejn.15139 [PubMed] [PMC]
Myers B, Greenwood-Van Meerveld B (2010) Divergent effects of amygdala glucocorticoid and mineralocorticoid receptors in the regulation of visceral and somatic pain. American Journal of Physiology. Gastrointestinal and Liver Physiology 298(2): G295–G303. https://doi.org/10.1152/ajpgi.00298.2009 [PubMed]
Oakley RH, Riddick NV, Moy SS, Cidlowski JA (2023) Imbalanced glucocorticoid and mineralocorticoid stress hormone receptor function has sex-dependent and independent regulatory effects in the mouse hippocampus. Neurobiology Stress 28: 100589. https://doi.org/10.1016/j.ynstr.2023.100589 [PubMed] [PMC]
Pesarico AP, Birmann PT, Pinto R, Padilha NB, Lenardão EJ, Savegnago L (2020) Short- and long-term repeated forced swim stress induce depressive-like phenotype in mice: Effectiveness of 3-[(4-chlorophenyl)selanyl]-1-methyl-1h-indole. Frontiers in Behavioral Neuroscience 14: 140. https://doi.org/10.3389/fnbeh.2020.00140 [PubMed] [PMC]
Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: A new animal model sensitive to antidepressant treatments. Nature 266(5604): 730–732. https://doi.org/10.1038/266730a0 [PubMed]
Ruiz-Sánchez E, López-Ramírez AM, Ruiz-Chow Á, Calvillo M, Reséndiz-Albor AA, Anguiano B, Rojas P (2021) Variability in behavioral phenotypes after forced swimming-induced stress in rats is associated with expression of the glucocorticoid receptor, nurr1, and IL-1β in the hippocampus. International Journal of Molecular Sciences 22(23): 12700. https://doi.org/10.3390/ijms222312700 [PubMed] [PMC]
Vafaei AA, Rashidy-Pour A, Taherian AA. (2008) Peripheral injection of dexamethasone modulates anxiety related behaviors in mice: an interaction with opioidergic neurons. Pakistan Journal of Pharmaceutical Sciences 21(3): 285–289. [PubMed]
Wei Q, Lu XY, Liu L, Schafer G, Shieh KR, Burke S, Robinson TE, Watson SJ, Seasholtz AF, Akil H (2004) Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proceedings of the National Academy of Sciences of the United States of America 101(32): 11851–11856. https://doi.org/10.1073/pnas.0402208101[PubMed] [PMC]
Zhao Y, Ma R, Shen J, Su H, Xing D, Du L (2008) A mouse model of depression induced by repeated corticosterone injections. European Journal of Pharmacology 581(1-2): 113–120. https://doi.org/10.1016/j.ejphar.2007.12.005 [PubMed]
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Kudryashov NV, Gorbunov AA, Mironov SE, Tikhonov DA, Nedorubov AA, Arshinova OYu, Simukhina MA, Busol VN, Fisenko VP

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

