Spontaneous remyelination following dimethyl sulfoxide-induced demyelination is accompanied by behavioral and neurological alteration in mice
DOI:
https://doi.org/10.18413/rrpharmacology.9.10059Abstract
Introduction: Dimethyl sulfoxide (DMSO) is a commonly used solvent that can be applied in experimental studies for preparation of hydrophobic solutions as well as in capacity of a cryopreservative in transplantology. According to modern data acquired from in vitro experiments, DMSO is able to change the structure of myelin by decreasing synthesis of its main components and inhibiting oligodendrocyte genesis.
Aim of the study: We studied influence of DMSO on anxiety and compulsive-like behavior, pain perception, motor coordination and myelin quantity in the corpus callosum of the C57BL/6 mice brain after prolonged oral administration of the solvent and 4 weeks after administration was stopped.
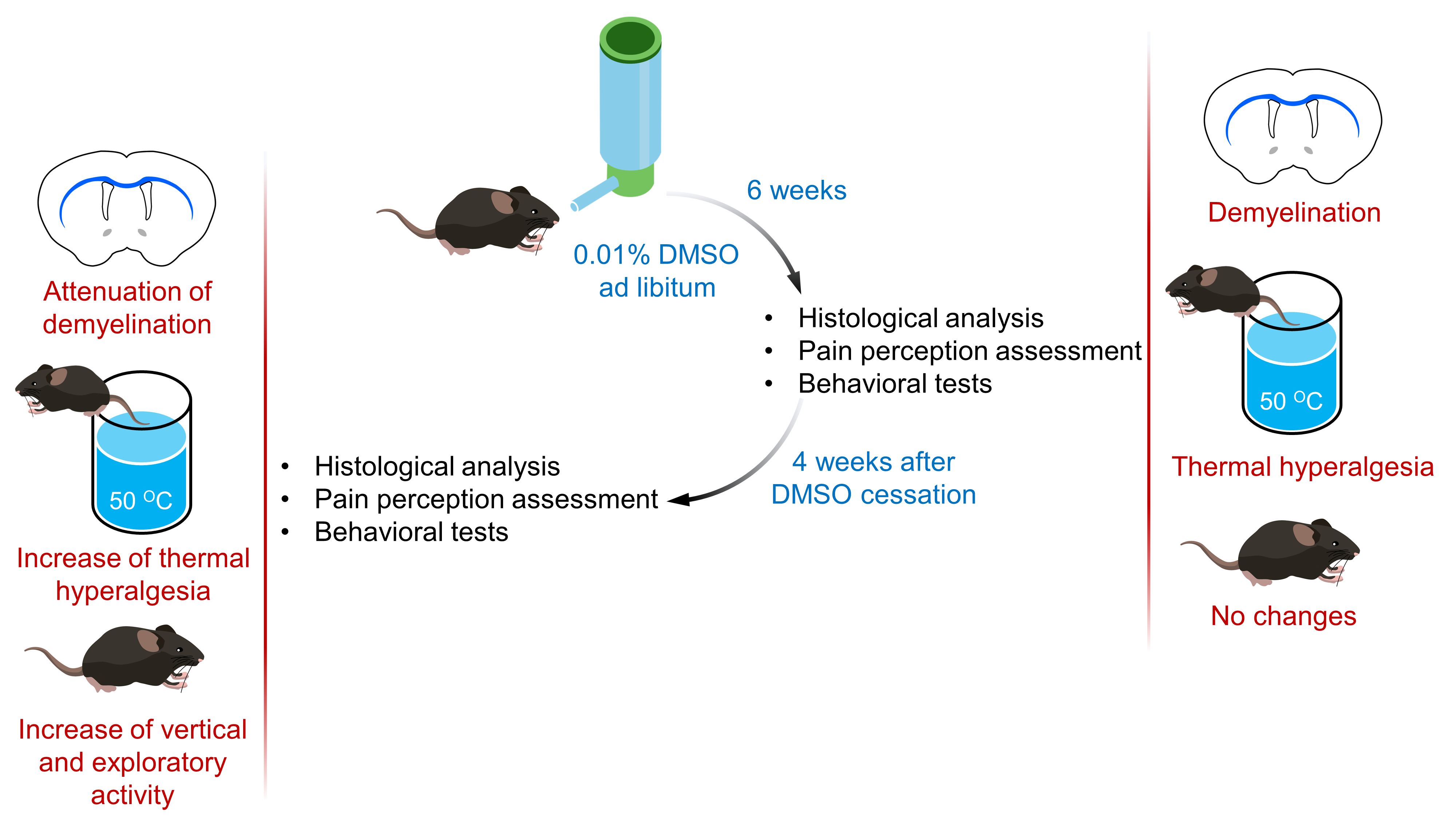
Materials and Methods: All the experiments were conducted on male inbreed C57BL/6 mice. DMSO was added to drinking water to achieve 0.01% concentration, and the obtained solution was administered ad libitum for 6 weeks. After 6 weeks of administration of DMSO and 4 weeks after administration of DMSO was stopped, anxiety-like behavior in open field test, compulsive-like behavior in marble burying test, motor coordination in rotarod test, pain perception in tail-immersion test, as well as myelin quantity in the corpus callosum were evaluated.
Results: It was established that DMSO consumed for 6 weeks was associated with decrease in the myelin quantity in thecorpus callosum and thermal hyperalgesia in tail-immersion test. During 4-week period after DMSO administration was stopped, attenuation of demyelination was observed, followed by an increase in thermal hyperalgesia in tail-immersion test, as well as vertical locomotion and exploratory activity in open field test.
Conclusions: 6-week ad libitum administration of 0.01% DMSO solution was associated with demyelination in corpus callosum of С57BL/6 mice, followed by thermal hyperalgesia. Cessation of DMSO led to spontaneous remyelination with an increase in thermal hyperalgesia, vertical locomotion and exploratory activity of mice.
Graphical Abstract
Keywords:
demyelination, dimethyl sulfoxide, hyperalgesia, mice, remyelinationReferences
Abdelkefi A, Lakhal A, Moojat N, Hamed LB, Fekih J, Ladeb S, Torjman L, Othman TB (2009) Severe neurotoxicity associated with dimethyl sulphoxide following PBSCT. Bone Marrow Transplantation 44(5): 323–324. https://doi.org/10.1038/bmt.2009.13 [PubMed]
Caldwell M, Ayo-Jibunoh V, Mendoza JC, Brimblecombe KR, Reynolds LM, Zhu Jiang XY, Alarcon C, Fiore E, J NT, Phillips GR, Mingote S, Flores C, Casaccia P, Liu J, Cragg SJ, McCloskey DP, Yetnikoff L (2023) Axo-glial interactions between midbrain dopamine neurons and oligodendrocyte lineage cells in the anterior corpus callosum. Brain Structureure and and Functionion 228(8): 1993–2006. https://doi.org/10.1007/s00429-023-02695-y [PubMed] [PMC]
Chang H, Liu J, Zhang Y, Wang F, Wu Y, Zhang L, Ai H, Chen G, Yin L (2017) Increased central dopaminergic activity might be involved in the behavioral abnormality of cuprizone exposure mice. Behavioural Brain Research 331: 143–150. https://doi.org/10.1016/j.bbr.2017.05.045 [PubMed]
Ding S, Gu Y, Cai Y, Cai M, Yang T, Bao S, Shen W, Ni X, Chen G, Xing L (2020) Integrative systems and functional analyses reveal a role of dopaminergic signaling in myelin pathogenesis. Journal of Translational Medicine 18(1): 109.https://doi.org/10.1186/s12967-020-02276-1 [PubMed] [PMC]
Jakkamsetti V, Scudder W, Kathote G, Ma Q, Angulo G, Dobariya A, Rosenberg RN, Beutler B, Pascual JM (2021) Quantification of early learning and movement sub-structure predictive of motor performance. Scientific Reports 11(1): 14405. https://doi.org/10.1038/s41598-021-93944–9 [PubMed] [PMC ]
Kaye TS, Egorin MJ, Riggs CE, Jr., Olman EA, Chou FT, Salcman M (1983) The plasma pharmacokinetics and tissue distribution of dimethyl sulfoxide in mice. Life Sciences 33(13): 1223–1230. https://doi.org/10.1016/0024-3205(83)90002-4 [PubMed]
Kudryashov NV, Gorbunov AA, Mironov SE, Tikhonov DA, Sviridkina NB, Tarasov VV, Fisenko VP (2022) The effect of dimethyl sulfoxide on behavior of c57bl/6 mice. Eksperimental'naya i Klinicheskaya Farmakologiya 85: 3–6. https://doi.org/10.30906/0869-2092-2022-85-9-3-6
Lubrich C, Giesler P, Kipp M (2022) Motor behavioral deficits in the cuprizone model: Validity of the rotarod test paradigm. International Journal of Molecular Sciences. 23(19): 11342. https://doi.org/10.3390/ijms231911342 [PubMed] [PMC]
O'Sullivan A, Lange S, Rotheneichner P, Bieler L, Aigner L, Rivera FJ, Couillard-Despres S (2019) Dimethylsulfoxide inhibits oligodendrocyte fate choice of adult neural stem and progenitor cells. Frontiers in Neuroscience 13: 1242. https://doi.org/10.3389/fnins.2019.01242 [PubMed] [PMC]
Palavra F, Viana SD, Henriques S, Dinis J, Martins J, Madeira MH, Santiago R, Petrella L, Sereno J, Castelo-Branco M, Pereira FC, Almeida L, Ambrosio AF, Reis F (2022) Defining milestones for the study of remyelination using the cuprizone mouse model: How early is early? Multiple Sclerosis and Related Disorders 63: 103886. https://doi.org/10.1016/j.msard.2022.103886 [PubMed]
Richter JS (2013) The effect of dopamine and its agonist pramipexole on oligodendrocytes in culture and in the cuprizone mouse model. PhD thesis, Göttingen, Germany: University of Göttingen.
Shieh KR, Yang SC (2020) Formosan wood mice (Apodemus semotus) exhibit more exploratory behaviors and central dopaminergic activities than C57BL/6 mice in the open field test. The Chinese Journal of Physiology 63(1): 27–34. https://doi.org/10.4103/CJP.CJP_47_19 [PubMed]
Sutrina SL, Lue NF, Chen GL, Chen WW (1987) Effect of dimethyl sulfoxide on transformed rat Schwann cells. Biochimica et Biophysica Acta 923(3): 451–462. https://doi.org/10.1016/0304-4165(87)90054-7 [PubMed]
Tamagnini F, Scullion S, Brown JT, Randall AD (2014) Low concentrations of the solvent dimethyl sulphoxide alter intrinsic excitability properties of cortical and hippocampal pyramidal cells. PLoS One 9(3): e92557. https://doi.org/10.1371/journal.pone.0092557 [PubMed] [PMC]
Thorburn KC, Paylor JW, Webber CA, Winship IR, Kerr BJ (2016) Facial hypersensitivity and trigeminal pathology in mice with experimental autoimmune encephalomyelitis. Pain 157(3): 627–642. https://doi.org/10.1097/j.pain.0000000000000409 [PubMed]
Tsukahara R, Yamamoto S, Yoshikawa K, Gotoh M, Tsukahara T, Neyama H, Ishii S, Akahoshi N, Yanagida K, Sumida H, Araki M, Araki K, Yamamura KI, Murakami-Murofushi K, Ueda H (2018) LPA5 signaling is involved in multiple sclerosis-mediated neuropathic pain in the cuprizone mouse model. Journal of Pharmacological Sciences 136(2): 93–96. https://doi.org/10.1016/j.jphs.2018.01.001 [PubMed]
Udell ME, Ni J, Garcia Martinez A, Mulligan MK, Redei EE, Chen H (2021) TailTimer: A device for automating data collection in the rodent tail immersion assay. PLoS One 16(8): e0256264. https://doi.org/10.1371/journal.pone.0256264[PubMed] [PMC]
Xu H, Yang HJ, McConomy B, Browning R, Li XM (2010) Behavioral and neurobiological changes in C57BL/6 mouse exposed to cuprizone: Effects of antipsychotics. Frontiers in Behavioral Neuroscience 4: 8. https://doi.org/10.3389/fnbeh.2010.00008 [PubMed] [PMC]
Yu Q, Hui R, Park J, Huang Y, Kusnecov AW, Dreyfus CF, Zhou R (2017) Strain differences in cuprizone induced demyelination. Cell and Bioscience 7: 59. https://doi.org/10.1186/s13578-017-0181-3 [PubMed] [PMC]
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Никита В. Кудряшов, Александр А. Горбунов, Надежда Б. Свиридкина, Сергей Е. Миронов, Дмитрий А. Тихонов, Андрей А. Недорубов, Владимир П. Фисенко

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

