Система эндогенных нейростероидов и ее роль в патогенезе и терапии психических расстройств
DOI:
https://doi.org/10.18413/rrpharmacology.9.10015Аннотация
Введение. Несмотря на то, что нейростероиды были открыты сравнительно недавно, очевидна их значимая роль в нейрохимических процессах и патогенезе ряда психиатрических и неврологических расстройств. Прежде всего, представляется важным уточнить определение эндогенных нейростероидов как класса стероидов, учитывая вариативность подходов к их описанию. В настоящее время к нейростероидам относят эндогенные стероиды, синтезируемые в центральной нервной системе, гонадах или надпочечниках и взаимодействующие с ГАМК, NMDA и сигма-1 рецепторами.
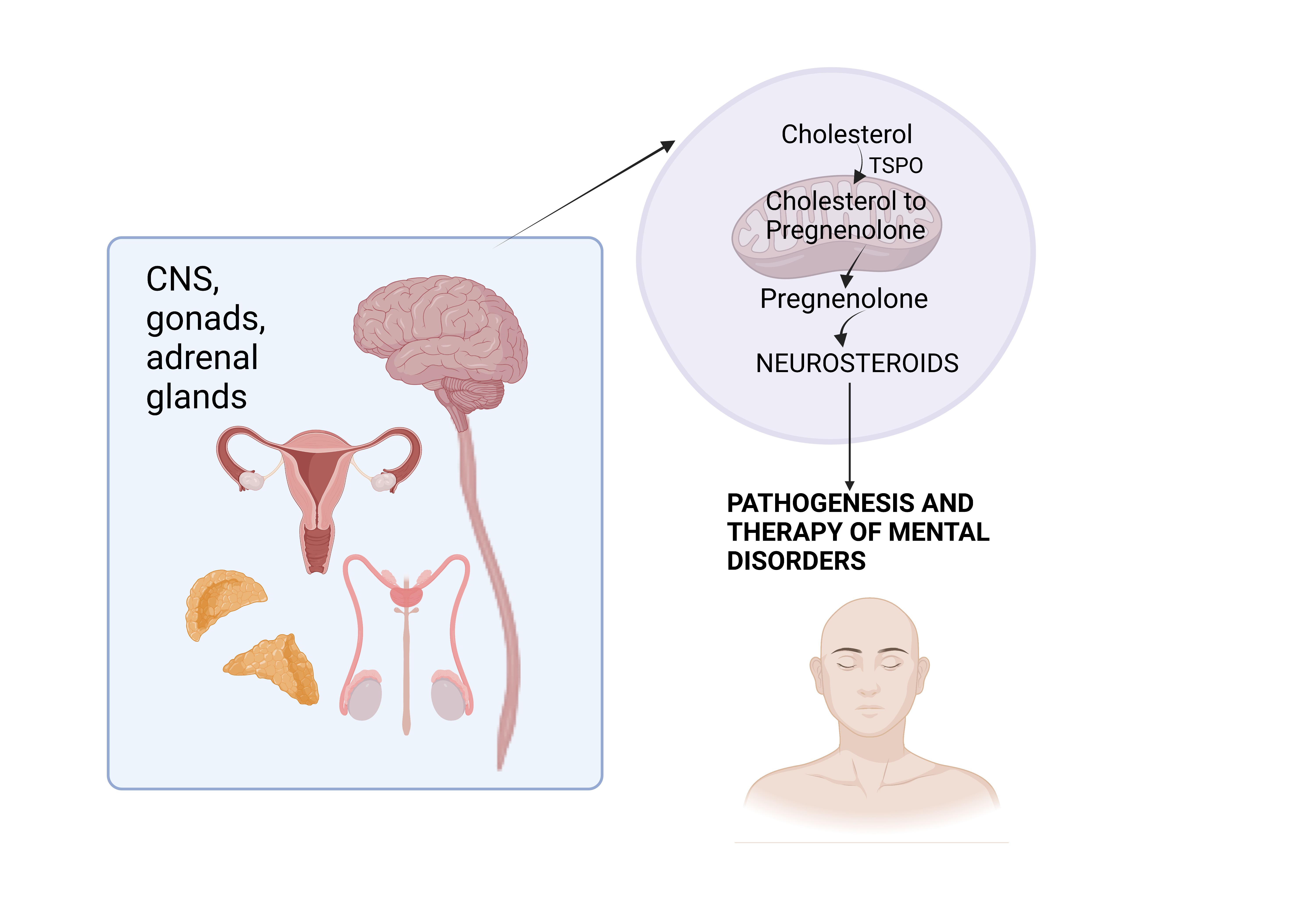
Биосинтез нейростероидов. Биосинтез нейростероидов начинается со скорость-лимитирующей реакции связывания TSPO с холестерином и транспортировки его в митохондрию, где под действием цитохрома P450scc (CYP11A1) синтезируется прегненолон.
Взаимодействие с ГАМКА, NMDA и сигма-1 рецепторами. Согласно экспериментальным данным, нейростероиды действуют как наиболее мощные эндогенные аллостерические модуляторы рецептора ГАМК, некоторые нейростероиды (ALLO, DHEA и др.) могут оказывать быстрое анксиолитическое и противосудорожное действие. Имеются некоторые экспериментальные данные об антипсихотических эффектах некоторых нейростероидов, реализуемых через NMDA-рецепторы: интрацеребральное введение ALLO лабораторным животным может предотвратить дальнейшее появление моторного возбуждения и других эквивалентов психоза после введения высоких доз амфетаминов. Также в нескольких исследованиях на животных моделях было доказано, что нейростероиды оказывают анксиолитическое действие через сигма-1 рецепторы.
Заключение. В статье описан процесс нейростероидогенеза и влияния нейростероидов на перечисленные рецепторы в соответствии с уже имеющимися научными данными. Кроме того, в статье описана специфическая роль различных нейростероидов в развитии психических заболеваний, включая тревожные расстройства, депрессию и шизофрению.
Графическая аннотация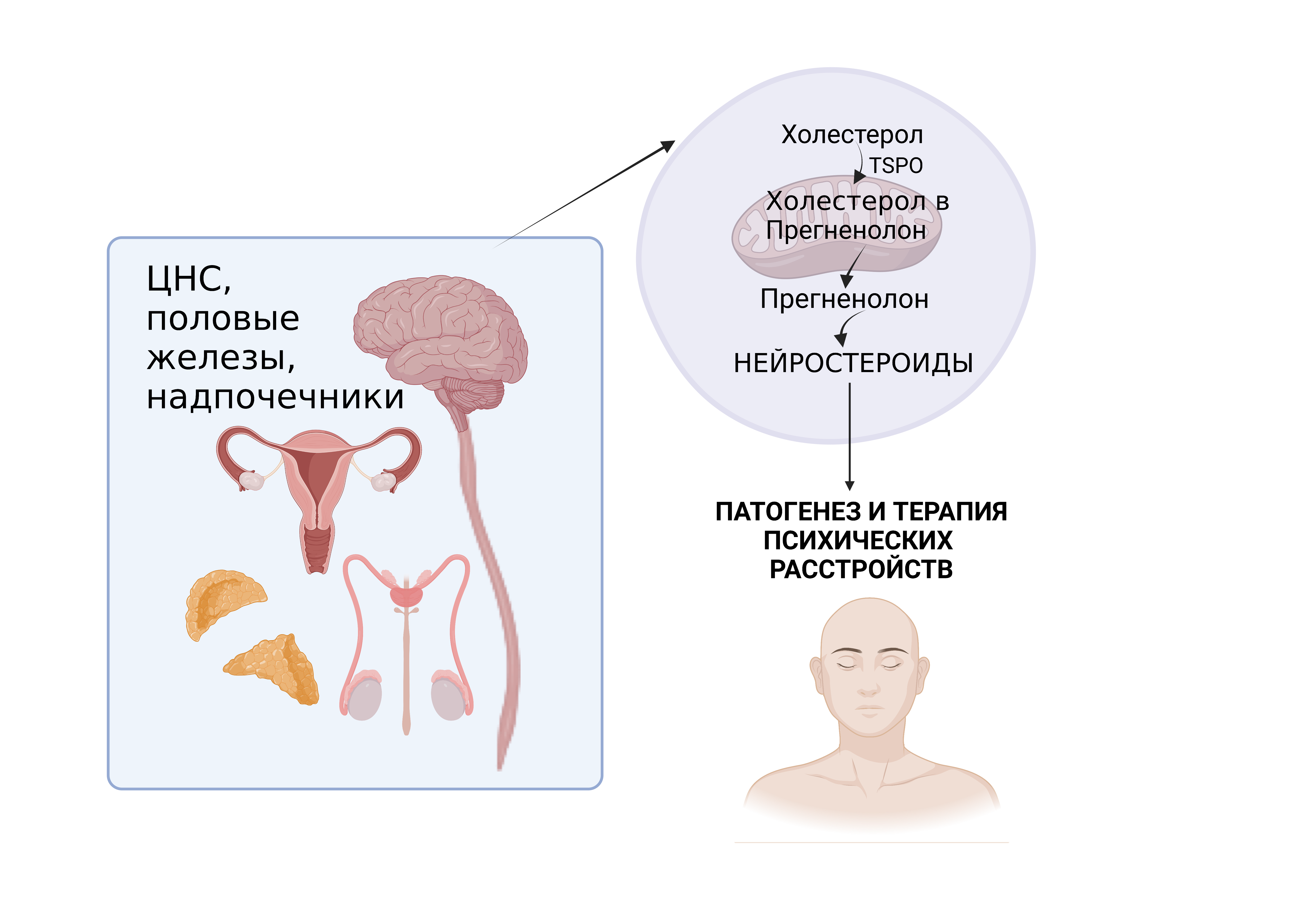
Графическая аннотация
Ключевые слова:
аллопрегнанолон, ГАМКА-рецепторы, DHEA, NMDA-рецепторы, прегненолон, сигма-1 рецепторы, стероидогенез, TSPOБиблиографические ссылки
Adamusová E, Cais O, Vyklický V, Kudová E, Chodounská H, Horák M, Vyklický L Jr (2013) Pregnenolone sulfate activates NMDA receptor channels. Physiological Research 62(6): 731–736. https://doi.org/10.33549/physiolres.932558 [PubMed]
Banks WA (2012) Brain meets body: the blood-brain barrier as an endocrine interface. Endocrinology 153(9): 4111–4119. https://doi.org/10.1210/en.2012-1435 [PubMed] [PMC]
Bergeron R, de Montigny C, Debonnel G (1996) Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: effects mediated via sigma receptors. The Journal of Neuroscience 16(3): 1193–1202. https://doi.org/10.1523/JNEUROSCI.16-03-01193.1996 [PubMed] [PMC]
Brickley SG, Mody I (2012) Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron 73(1): 23–34. https://doi.org/10.1016/j.neuron.2011.12.012 [PubMed] [PMC]
Calderon-Dominguez M, Gil G, Medina MA, Pandak WM, Rodríguez-Agudo D (2014) The StarD4 subfamily of steroidogenic acute regulatory-related lipid transfer (START) domain proteins: new players in cholesterol metabolism. The International Journal of Biochemistry & Cell Biology 49: 64–68. https://doi.org/10.1016/j.biocel.2014.01.002 [PubMed] [PMC]
Cannon TD (2015) How schizophrenia develops: cognitive and brain mechanisms underlying onset of psychosis. Trends in Cognitive Sciences 19(12): 744–756. https://doi.org/10.1016/j.tics.2015.09.009 [PubMed] [PMC]
Compagnone NA, Mellon SH (2000) Neurosteroids: biosynthesis and function of these novel neuromodulators. Frontiers Neuroendocrinology 1(1): 1– https://doi.org/10.1006/frne.1999.0188 [PubMed]
Corpéchot C, Robel P, Axelson M, Sjövall J, Baulieu EE (1981) Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proceedings of the National Academy of Sciences of the United States of America 78(8): 4704– https://doi.org/10.1073/pnas.78.8.4704 [PubMed]
Costa E, Cheney DL, Grayson DR, Korneyev A, Longone P, Pani L, Romeo E, Zivkovich E, Guidotti A (1994) Pharmacology of neurosteroid biosynthesis. Role of the mitochondrial DBI receptor (MDR) complex. Annals of the New York Academy of Sciences 746: 223–242. https://doi.org/10.1111/j.1749-6632.1994.tb39240.x [PubMed]
Diotel N, Charlier TD, Lefebvre d'Hellencourt C, Couret D, Trudeau VL, Nicolau JC, Meilhac O, Kah O, Pellegrini E (2018) Steroid transport, local synthesis, and signaling within the brain: roles in neurogenesis, neuroprotection, and sexual behaviors. Frontiers in Neuroscience 12: 84. https://doi.org/10.3389/fnins.2018.00084 [PubMed] [PMC]
Do Rego JL, Seong JY, Burel D, Leprince J, Vaudry D, Luu-The V, Tonon MC, Tsutsui K, Pelletier G, Vaudry H (2012) Regulation of neurosteroid biosynthesis by neurotransmitters and neuropeptides. Frontiers in Endocrinology 3: 4. https://doi.org/10.3389/fendo.2012.00004 [PubMed] [PMC]
Elustondo P, Martin LA, Karten B (2017) Mitochondrial cholesterol import. Biochimica et Biophysica Acta. Molecular and Cell Biology of Lipids 1862(1): 90–101. https://doi.org/10.1016/j.bbalip.2016.08.012 [PubMed]
Flood JF, Morley JE, Roberts E (1992) Memory-enhancing effects in male mice of pregnenolone and steroids metabolically derived from it. Proceedings of the National Academy of Sciences of the United States of America 89(5): 1567–1571. https://doi.org/10.1073/pnas.89.5.1567 [PubMed] [PMC]
Hashimoto K (2015) Activation of sigma-1 receptor chaperone in the treatment of neuropsychiatric diseases and its clinical implication. Journal of Pharmacological Science 127(1): 6–9. https://doi.org/10.1016/j.jphs.2014.11.010[PubMed]
Hayashi T, Su TP (2010) Cholesterol at the endoplasmic reticulum: roles of the sigma-1 receptor chaperone and implications thereof in human diseases. Sub-Cellular Biochemistry 51: 381– https://doi.org/10.1007/978-90-481-8622-8_13 [PubMed] [PMC]
Joshi S, Kapur J (2019) Neurosteroid regulation of GABAA receptors: A role in catamenial epilepsy. Brain Research 1703: 31–40. https://doi.org/10.1016/j.brainres.2018.02.031 [PubMed] [PMC]
Kalinina TS, Shimshirt AA, Kudriashov NV, Voronina TA, Seredenin SB (2014) Neurosteroidogenesis and exploratory responses in rodents. Experimental and Clinical Pharmacology [Eksperimental'naia i Klinicheskaia Farmakologiia] 77(2): 3–7. https://pubmed.ncbi.nlm.nih.gov/24791332/ [PubMed] [in Russian]
Khvostova EP, Pustylnyak VO, Korbut AI, Otpuschennikov AA, Gulyaeva LF, Krylova IN (2012) Modern approach to molecular characterization of prostate tumors. Acta Biomedica Scientifica 3(1): 83–88. [in Russian]
Kraemer FB, Shen WJ, Azhar S (2017) SNAREs and cholesterol movement for steroidogenesis. Molecular and Cellular Endocrinology 441: 17–21. https://doi.org/10.1016/j.mce.2016.07.034 [PubMed] [PMC]
Lambert J, Belelli D, Hill-Venning C, Peters J (1995) Neurosteroids and GABAA receptor function. Trends in Pharmacological Sciences 16(9): 295– https://doi.org/10.1016/S0165-6147(00)89058-6 [PubMed]
Larsen MC, Lee J, Jorgensen JS, Jefcoate CR (2020) STARD1 functions in mitochondrial cholesterol metabolism and nascent HDL formation. Gene expression and molecular mRNA imaging show novel splicing and a 1:1 mitochondrial association. Frontiers in Endocrinology 11: 559674. https://doi.org/10.3389/fendo.2020.559674[PubMed] [PMC]
Li Z, Cui S, Zhang Z, Zhou R, Ge Y, Sokabe M, Chen L (2009) DHEA-neuroprotection and –neurotoxicity after transient cerebral ischemia in rats. Journal of Cerebral Blood Flow and Metabolism 29(2): 287–296. https://doi.org/10.1038/jcbfm.2008.118 [PubMed]
Longone P, di Michele F, D'Agati E, Romeo E, Pasini A, Rupprecht R (2011) Neurosteroids as neuromodulators in the treatment of anxiety disorders. Frontiers in Endocrinology 2: 55. https://doi.org/10.3389/fendo.2011.00055[PubMed] [PMC]
Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM (1986) Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 232(4753): 1004– https://doi.org/10.1126/science.2422758 [PubMed]
Marriott KS, Prasad M, Thapliyal V, Bose HS (2012) σ-1 receptor at the mitochondrial-associated endoplasmic reticulum membrane is responsible for mitochondrial metabolic regulation. The Journal of Pharmacology and Experimental Therapeutics 343(3): 578– https://doi.org/10.1124/jpet.112.198168 [PubMed] [PMC]
McNeela AM, Bernick C, Hines RM, Hines DJ (2018) TSPO regulation in reactive gliotic diseases. Journal of Neuroscience Research 96(6): 978–988. https://doi.org/10.1002/jnr.24212 [PubMed]
Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE (1999) Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proceedings of the National Academy of Sciences of the United States of America 96(22): 12905–12910. https://doi.org/10.1073/pnas.96.22.12905 [PubMed] [PMC]
Monnet F, Maurice T (2006) The sigma1 protein as a target for the non-genomic effects of neuro(active)steroids: molecular, physiological, and behavioral aspects. Journal of Pharmacological Sciences 100(2): 93–118. https://doi.org/10.1254/jphs.cr0050032 [PubMed]
Noda Y, Kamei H, Kamei Y, Nagai T, Nishida M, Nabeshima T (2000) Neurosteroids ameliorate conditioned fear stress: an association with sigma receptors. Neuropsychopharmacology 23(3): 276–284. https://doi.org/10.1016/S0893-133X(00)00103-2 [PubMed]
Pabba M, Sibille E (2015) Sigma-1 and N-methyl-d-aspartate receptors: a partnership with beneficial outcomes. Molecular Neuropsychiatry 1(1): 47–51. https://doi.org/10.1159/000376549 [PubMed] [PMC]
Pabba M, Wong AY, Ahlskog N, Hristova E, Biscaro D, Nassrallah W, Ngsee JK, Snyder M, Beique JC, Bergeron R (2014) NMDA receptors are upregulated and trafficked to the plasma membrane after sigma-1 receptor activation in the rat hippocampus. The Journal of Neuroscience 34(34): 11325–11338. https://doi.org/10.1523/JNEUROSCI.0458-14.2014 [PubMed] [PMC]
Paul SM, Purdy RH (1992) Neuroactive steroids. FASEB Journal 6(6): 2311–2322. https://doi.org/10.1096/fasebj.6.6.1347506 [PubMed]
Porcello DM, Huntsman MM, Mihalek RM, Homanics GE, Huguenard JR (2003) Intact synaptic GABAergic inhibition and altered neurosteroid modulation of thalamic relay neurons in mice lacking delta subunit. Journal of Neurophysiology 89(3): 1378–1386. https://doi.org/10.1152/jn.00899.2002 [PubMed]
Rodriguez-Agudo D, Ren S, Hylemon PB, Montañez R, Redford K, Natarajan R, Medina MA, Gil G, Pandak WM (2006) Localization of StarD5 cholesterol binding protein. Journal of Lipid Research 47(6): 1168–1175. https://doi.org/10.1194/jlr.M500447-JLR200 [PubMed]
Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, Groyer G, Adams D, Schumacher M (2010) Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nature Reviews. Drug Discovery 9(12): 971–988. https://doi.org/10.1038/nrd3295 [PubMed]
Salman ED, Faye-Petersen O, Falany CN (2011) Hydroxysteroid sulfotransferase 2B1b expression and localization in normal human brain. Hormone Molecular Biology and Clinical Investigation 8(1): 445–454. https://doi.org/10.1515/HMBCI.2011.117 [PubMed] [PMC]
Schumacher M, Robel P, Baulieu E-E (2009) Neurosteroids. In: Squire LR (Ed.) Encyclopedia of Neuroscience, volume 1. Academic Press, Oxford, pp. 1015-1020.
Shu HJ, Eisenman LN, Jinadasa D, Covey DF, Zorumski CF, Mennerick S (2004) Slow actions of neuroactive steroids at GABAA receptors. The Journal of Neuroscience 24(30): 6667–https://doi.org/10.1523/JNEUROSCI.1399-04.2004 [PubMed] [PMC]
Sigel E (2002) Mapping of the benzodiazepine recognition site on GABA(A) receptors. Current Topics in Medicinal Chemistry 2(8): 833– https://doi.org/10.2174/1568026023393444 [PubMed]
Stell BM, Brickley SG, Tang CY, Farrant M, Mody I (2003) Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proceedings of the National Academy of Sciences of the United States of America 100(24): 14439– https://doi.org/10.1073/pnas.2435457100 [PubMed] [PMC]
Sugasawa Y, Cheng WW, Bracamontes JR, Chen ZW, Wang L, Germann AL, Pierce SR, Senneff TC, Krishnan K, Reichert DE, Covey DF, Akk G, Evers AS (2020) Site-specific effects of neurosteroids on GABAA receptor activation and desensitization. Elife 9: e55331. https://doi.org/10.7554/eLife.55331 [PubMed] [PMC]
van Broekhoven F, Verkes RJ (2003) Neurosteroids in depression: a review. Psychopharmacology 165(2): 97–110. https://doi.org/10.1007/s00213-002-1257-1 [PubMed]
Vyklicky V, Korinek M, Smejkalova T, Balik A, Krausova B, Kaniakova M, Lichnerova K, Cerny J, Krusek J, Dittert I, Horak M, Vyklicky L (2014) Structure, function, and pharmacology of NMDA receptor channels. Physiological Research 63(Suppl 1): S191–S203. https://doi.org/10.33549/physiolres.932678 [PubMed]
Wang M (2011) Neurosteroids and GABA-A receptor function. Frontiers in Endocrinology 2: 44. https://doi.org/10.3389/fendo.2011.00044 [PubMed] [PMC]
Wang MD (2008) Neurosteroid: Molecular Mechanisms of Action on the GABAA Receptor. In: Ritsner MS, Weizman A (Eds) Neuroactive Steroids in Brain Function, Behavior and Neuropsychiatric Disorders. Springer, Dordrecht, 564 p. https://doi.org/10.1007/978-1-4020-6854-6
Witt KA, Sandoval KE (2014) Steroids and the blood-brain barrier: therapeutic implications. Advances in Pharmacology 71: 361–390. https://doi.org/10.1016/bs.apha.2014.06.018 [PubMed]
Zhemkov V, Liou J, Bezprozvanny I (2021) Sigma 1 receptor, cholesterol and endoplasmic reticulum contact sites. Contact 4: 10.1177/25152564211026505. https://doi.org/1177/25152564211026505
Загрузки
Опубликован
Как цитировать
Выпуск
Раздел
Лицензия
Copyright (c) 2023 Research Results in Pharmacology

Это произведение доступно по лицензии Creative Commons «Attribution» («Атрибуция») 4.0 Всемирная.
 Русский
Русский
 English
English

