Predictive analysis and prediction of the main molecular targets for the N-acetyl-6-aminohexanoate derivative
DOI:
https://doi.org/10.18413/rrpharmacology.11.560Аннотация
Introduction: N-acetyl-6-aminohexanoate (acexamic acid) and its derivatives are actively studied as promising compounds for the creation of new drugs, but their pharmacokinetic parameters and detailed mechanisms underlying a wide range of biochemical activities are still unclear.
Materials and Methods: PASS Online, Molinspiration Property Calculation Service and OSIRIS Property Explorer were used for predictive analysis. To determine the molecular biomarkers of the N-acetyl-6-aminohexanoate derivative – 2-ethyl-6-methyl-3-hydroxypyridinium N-acetylhexanoic acid, ADMET (Absorption, Distribution, Metabolism, Excretion, Toxicity) parameters were predicted. Molecular docking was performed using GalaxyWEB Sagittarius service followed by evaluation of the results using RCSB Protein Data Bank and UniProt Consortium databases.
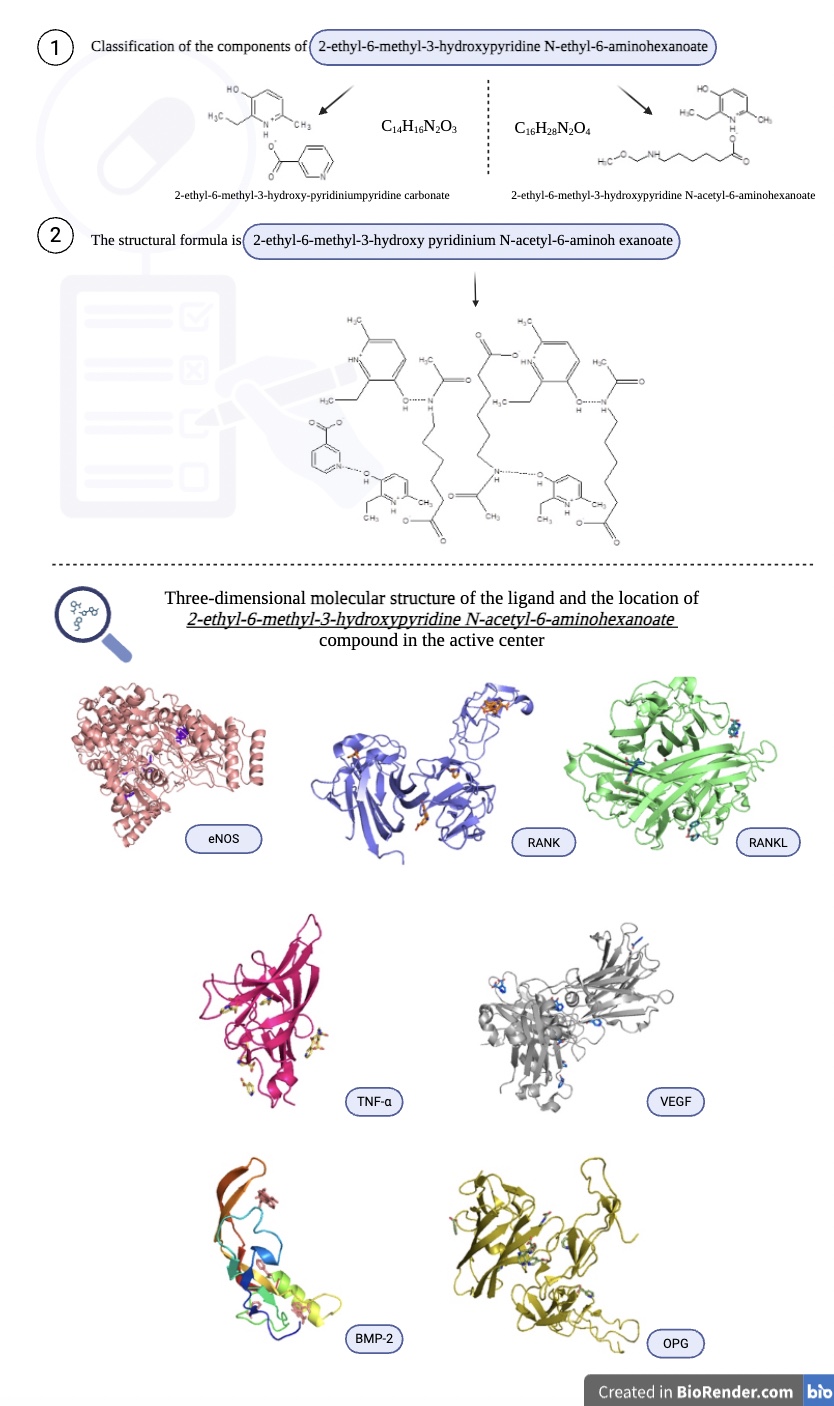
Results: The study of the pharmacokinetic properties of 2-ethyl-6-methyl-3-hydroxypyridine N-acetyl-6-aminohexanoate revealed its potential suitability as a promising drug with high bioavailability. The highest degree of affinity is predicted with the eNOS center – binding energy from -7.2 to -8.3 kcal/mol; the VEGF center – binding energies from -6.1 to -7.7 kcal /mol, and the RANKL centre – binding energies from - 6.0 - 6,9 kcal mol.
Conclusion: The results of predictor analysis and molecular docking suggest that the N-acetyl-6-aminohexanoate-2-ethyl-6-methyl-3-hydroxypyridinium derivative is a safe and promising compound. Potential biotargets include eNOS, VEGF, and RANKL.
Графическая аннотация
Ключевые слова:
molecular docking, drug discovery, 2-ethyl-6-methyl-3-hydroxypyridinium N-acetyl-6-aminohexanoate, eNOS, VEGF, RANKLБиблиографические ссылки
Alkhatatneh BA, Pakhomov DV, Pronina EN, Blinova EV, Kilmyashkina MF, Kazaeva MA (2020) Study of the pharmacological activity of silver salt of acexamic acid in the treatment of burn wounds. Bulletin “Biomedicine and Sociology” [Vestnik “Biomeditsina i Sotsiologiya”] 5(2): 63–70. http://dx.doi.org/10.26787/nydha-2618-8783-2020-5-2-63-70 [in Russian]
Alkhatatneh Bashar AS (2023) Regenerative and cosmetic effects of topical dosage forms of acexamic acid salts in burn lesions of the skin (experimental study), PhD thesis. Ogarev Mordovia State University, Saransk, Russia, 23 pp. [in Russian]
Andrianova EV (2023) Biochemical aspects of the pro-regenerative action of a new derivative of N-acetyl-6-aminohexanoic acid. PhD thesis, Federal Research Center for Nutrition, Biotechnology and Food Safety, Moscow, Russia, 23 pp. [in Russian]
Andrianova EV, Egorova EN (2019) Acexamic acid derivatives and their biochemical effects. Bulletin of Tver State University. Series: Chemistry [Vestnik Tverskogo Gosudarstvennogo Universiteta. Seriya: Khimiya] 1(35): 164–169. [in Russian]
Bhardwaj A, Swe KMM, Sinha NK (2023) Treatment for osteoporosis in people with beta-thalassaemia. Cochrane Database of Systematic Reviews 5(5): CD010429. https://doi.org/10.1002/14651858.CD010429.pub3 [PubMed] [PMC]
Blinova E, Pakhomov D, Shimanovsky D, Kilmyashkina M, Mazov Y, Demura T, Drozdov V, Blinov D, Deryabina O, Samishina E, Butenko A, Skachilova S, Sokolov A, Vasilkina O, Alkhatatneh BA, Vavilova O, Sukhov A, Shmatok D, Sorokvasha I, Tumutolova O, Lobanova E (2021) Cerium-containing N-acetyl-6-aminohexanoic acid formulation accelerates wound reparation in diabetic animals. Biomolecules 11(6): 834. https://doi.org/10.3390/biom11060834 [PubMed] [PMC]
Bogomolova OA, Demidova MA, Skachilova SY (2018) A derivative of 3-hydroxypyridine with anxiolytic and nootropic activity: patent 2 664 453 Russian Federation: IPC A61K 31/4425 (2018.05); C07D 213/18 (2018.05); A61P 25/22 (2018.05); A61P 25/28 (2018.05); patent holder ”Tver State Medical University” of the Ministry of Health of the Russian Federation. No. 2017141094, appl. 24.11.2017, publ. 17.08.2018, Bull. No. 23, 18 p. [in Russian]
Danilenko AP, Zhunusov NS, Khentov AA, Pokrovsky VM, Kuznetsov AV, Kulikov AL, Nadezhdin SV, Danilenko LM, Peresypkina AA, Loboda YaV, Gudyrev OS, Rubanov MYu, Avtina TV (2024) Pharmacokinetic properties of a new supramolecular complex based on 2-ethyl-6-methyl-3-hydroxypyridinium N-acetyl-6-aminohexanoate and 2-ethyl-6-methyl-3-hydroxypyridinium 3-pyridinocarbonoate, which has osteoprotective activity in in vitro studies. Experimental and Clinical Pharmacology [Eksperimental’naya i Klinicheskaya Farmakologiya] 87(2): 13–19. https://doi.org/10.30906/0869-2092-2024-87-2-13-19 [in Russian]
Danilenko LM, Korokin MV, Pokrovsky MV, Danilenko AP, Khentov AA (2023) Evaluation of possible mechanisms of osteoprotective action of a supramolecular complex based on 3-oxypyridine derivatives in steroid-induced osteoporosis. Experimental and Clinical Pharmacology [Eksperimental’naya i Klinicheskaya Farmakologiya] 86(115): 47. https://doi.org/10.30906/ekf-2023-86s-47 [in Russian]
Hadj Abdallah N, Baulies A, Bouhlel A, Bejaoui M, Zaouali MA, Ben Mimouna S, Messaoudi I, Fernandez-Checa JC, García Ruiz C, Ben Abdennebi H (2018) The effect of zinc acexamate on oxidative stress, inflammation and mitochondria induced apoptosis in rat model of renal warm ischemia. Biomedicine & Pharmacotherapy 105: 573–58. https://doi.org/10.1016/j.biopha.2018.06.017 [PubMed]
Klinaku FT, Comi L, Giglione C, Magni P (2025) An integrated view of the pathophysiological crosstalk between adipose tissue, bone and cardiovascular system in men and women. Journal of Endocrinological Investigation 48(5): 1061–1074. https://doi.org/10.1007/s40618-024-02516-x [PubMed]
Kravchenko AD, Pyatigorskaya NV, Brkich GE, Yevsieieva LV, Kyrychenko AV, Kovalenko SM (2022) Synthesis, molecular docking, ADMET study and in vitro pharmacological research of 7-(2-chlorophenyl)-4-(4-methylthiazol-5-yl)-4,6,7,8-tetrahydroquinoline-2,5(1H,3H)-dione as a promising non-opioid analgesic drug. Research Results in Pharmacology 8(1): 1–11. https://doi.org/10.3897/rrpharmacology.8.80504
Malygin AS, Popov NS, Demidova MA, Marasanov SB (2018) Evaluation of the bioavailability of acetylaminohexanoic acid ethylthiazolylamide upon intragastric administration to rabbits. Pharmacokinetics and Pharmacodynamics [Farmakokinetika i Farmakodinamika] 1: 56–63. https://doi.org/10.24411/2587-7836-2018-10007 [in Russian]
Mazov YaA, Dmitriev AA, Pirozhkov AS, Blinov DS, Timoshkin DE, Termulaeva RM, Semeleva EV, Blinova EV (2023) Compound N-acetyl-6-aminohexanoic acid suppresses nerve conduction. Bulletin “Biomedicine and Sociology” [Vestnik “Biomeditsina i Sotsiologiya”] 8(3): 59–64. http://dx.doi.org/10.26787/nydha-2618-8783-2023-8-3 [in Russian]
Nguyen A, Lee P, Rodriguez EK, Chahal K, Freedman BR, Nazarian A (2025) Addressing the growing burden of musculoskeletal diseases in the ageing US population: challenges and innovations. The Lancet. Healthy Longevity 6(5): 100707.https://doi.org/10.1016/j.lanhl.2025.100707 [PubMed]
Pakhomov DV, Blinova EV, Shimanovsky DN, Kilmyashkina MF, Kazaeva MA, Blinov DS, Nelipa MV, Nikolaev AV, Alkhatatnekh BA, Skachilova SYa, Bogoyavlenskaya TA, Kytko OV (2020) Evidence-based aspects of stimulating uncomplicated wound healing with local application of silver salt of acexamic acid. Operative Surgery and Clinical Anatomy [Operativnaya Khirurgiya i Klinicheskaya Anatomiya] 4(1): 19–25. https://doi.org/10.17116/operhirurg2020401119 [in Russian]
Petrovskaya MA, Petrova MB, Egorova EN, Andrianova EV (2024) Morphological and biochemical features of regeneration of thermal skin burns in rats at application of 2-ethyl-6-methyl-3-hydroxypyridinium N-acetyl-6-aminohexanoate. Upper Volga Medical Journal [Verkhnevolzhskii Meditsinskii Zhurnal] 23(3): 18–22. [in Russian]
Polozova Anastasia V, Boyarinov Gennadii A, Nikolsky Viktor O, Zolotova Marina V, Deryugina Anna V (2021) The functional indexes of RBCs and microcirculation in the traumatic brain injury with the action of 2-ethil-6-methil-3-hydroxypiridin succinate. BMC Neuroscience 22(1): 57. https://doi.org/10.1186/s12868-021-00657-w [PubMed] [PMC]
Perez-Amodio S, Rubio N, Vila OF, Navarro-Requena C, Castaño O, Sanchez-Ferrero A, Marti-Munoz J, Alsina-Giber M, Blanco J, Engel E (2021) Polymeric composite dressings containing calcium-releasing nanoparticles accelerate wound healing in diabetic mice. Advances in Wound Care 10(6): 301–316 https://doi.org/10.1089/wound.2020.1206 [PubMed]
Shcheblykina OV, Shcheblykin DV, Trunov KS, Danilenko AP, Lipatov VS (2022) Experimental study of new derivatives of 3-hydroxypyridine as pharmacological agents for the correction of ischemic brain injury after intracerebral hemorrhage. Research Results in Pharmacology 8(1): 71–83. https://doi.org/10.3897/rrpharmacology.8.80378
Skachilova SYa, Ermakova GA, Blinova EV, Blinov DS, Proskurina OV, Shilova EV, Aleshina VA, Kilmyashkina MF, Mazov YaA, Sokolov AI, Zheltukhin NK, Simakina EA, Pakhomov DV, Shmatok DO (2023) Spray with wound healing and anti-inflammatory effects: patent 2 790 489 Russian Federation: IPC A61K 9/12 (2022.08); A61K 31/05 (2022.08); A61K 31/195 (2022.08); A61P 17/02 (2022.08); patent holder: Joint-Stock Company ”All-Union Scientific Center for Safety of Biologically Active Substances” (JSC ”VNC BAV”). No. 2021113416, appl. 12.05.2021, publ. 21.02.2023, Bull. No. 6, 11 p. [in Russian]
Sorg H, Sorg CGG (2023) Skin wound healing: Of players, patterns, and processes. European Surgical Research 64(2): 141–157. https://doi.org/10.1159/000528271 [PubMed]
Trunov KS, Danilenko AP, Skachilova SYa, Gudyrev OS, Danilenko LM, Pokrovsky MV, Simakina EA, Ermakova GA, Shilova EV, Nadezhdin SV, Khentov AA, Malorodova TN, Boeva EV, Cherednichenko AV, Shcheblykina OV, Miller ES (2023) Pharmacological compound based on 2-ethyl-6-methyl-3-hydroxypyridinium N-acetyl-6-aminohexanoate and 2-ethyl-6-methyl-3-hydroxypyridinium 3-pyridinocarbonoate and its use for the correction and prevention of osteoporosis: patent 2806116 Russian Federation: IPC A61K 31/195 (2006.01), A61K 31/4412 (2006.01), A61P 19/10 (2006.01); patent holder “Belgorod State National Research University” (National Research University “BelSU”). No. 2023102529, appl. 06.02.2023, publ. 26.10.2023, Bull. No. 30, 12 p. [in Russian]
Загрузки
Опубликован
Как цитировать
Выпуск
Раздел
Лицензия
Copyright (c) 2025 Danilenko AP, Khentov AA, Kuznetsov AV, Shmigerova VS, Stepenko YuV, Tarasova AP, Yakushev VI, Boeva EV, Miller AV, Tatarenkova IA, Puzanova TV, Loboda YaV, Trashchenko AP, Gudyrev OS, Motailo AL, Kuzichkin VN, Milashechko VN, Danilenko LM

Это произведение доступно по лицензии Creative Commons «Attribution» («Атрибуция») 4.0 Всемирная.
 Русский
Русский
 English
English

