Regulation of 11β-hydroxysteroid dehydrogenase isoforms: pharmacophore search and molecular design of prospective 11β-HSD1 inhibitors
DOI:
https://doi.org/10.18413/rrpharmacology.11.862Аннотация
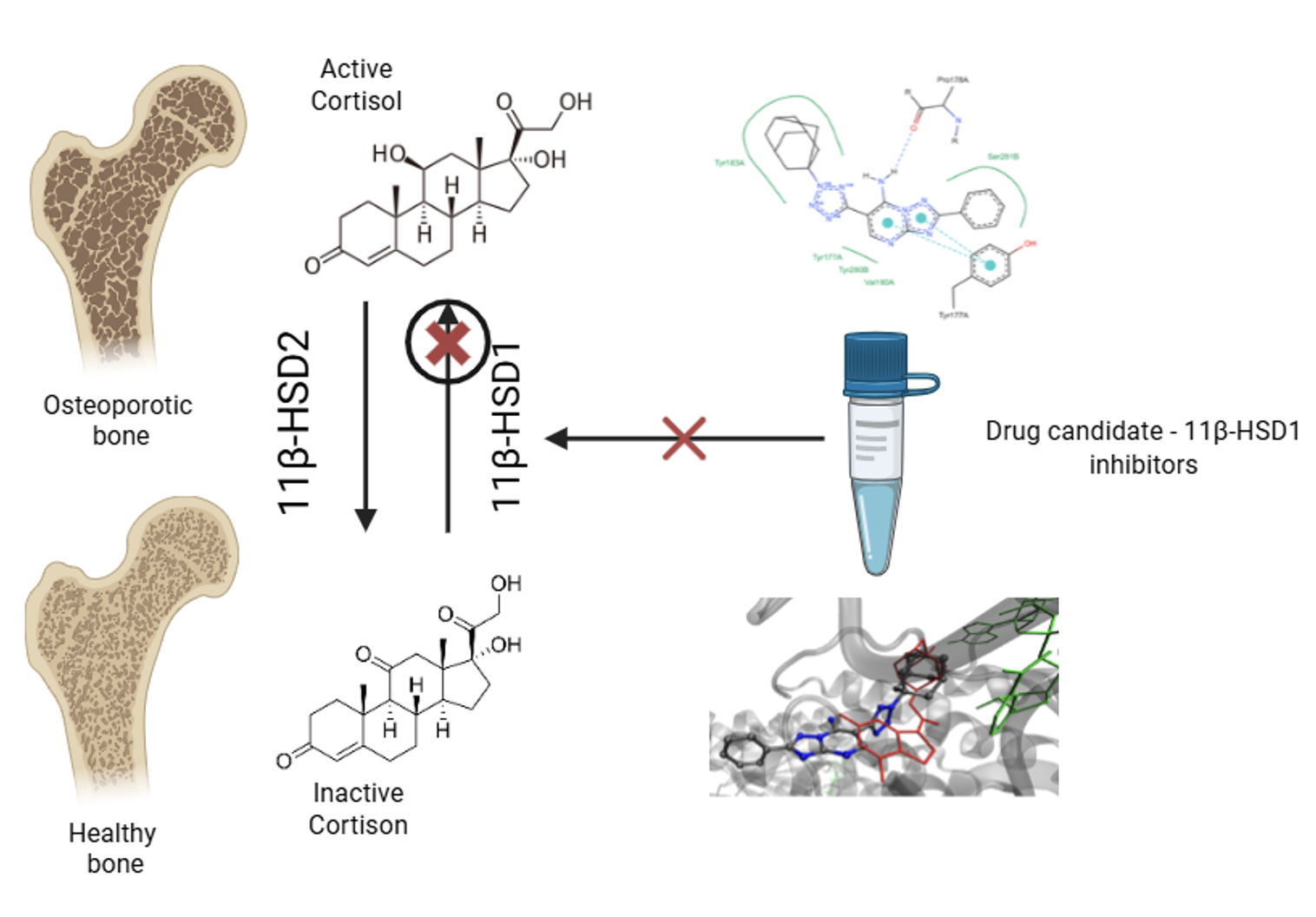
Introduction: Osteoporosis is an important medical and social public health problem in an aging or elderly society, the issue of pharmacological correction of which remains unresolved to this day.
Materials and Methods: A design of prospective 11β-HSD1 inhibitors was performed on the basis of the literature data. A virtual screening of the designed compounds was performed using pharmacophore searching (DataWarrior, Flexophore), pharmacophore alignment (PhESA), and molecular docking (Jamda). 2D protein-ligand interaction maps were generated using PoseEdit, and 3D visualizations were produced in VMD. The cytotoxicity of the compounds was assessed in HEK-293 cells across a concentration range of 8–1024 μM, with IC50 values calculated in RStudio.
Results and Discussion: A comprehensive virtual screening of prospective 11β-HSD1 inhibitors was performed, incorporating pharmacophore searching, PhESA alignment, and molecular docking against the 11β-HSD1 complex (PDB: 4YYZ). Several compounds (TS-897, TS-883, TS-945, and others) demonstrated Jamda Score values comparable to or exceeding that of the native ligand, indicating strong predicted binding affinity. Additional candidates were identified based on high pharmacophore similarity (>0.95). Among the 14 proposed compounds, four (IV-81, IV-91, IV-158) exhibited no cytotoxicity in HEK-293 cells (IC50 > 1024 µM). These findings support the potential of the synthesized azoloazine derivatives as novel 11β-HSD1 inhibitors for further in vitro and in vivo studies in osteoporosis therapy.
Conclusion: As a result of this work, original azoloazine derivatives have been developed for further biological testing aimed at regulating the activity of 11β-hydroxysteroid dehydrogenase isoforms (11β-HSDs) for pharmacological correction of bone remodeling and osteoreparation disorders.
Графическая аннотация
Ключевые слова:
osteoporosis, 11β-Hydroxysteroid Dehydrogenase, regulation, 11β-HSD inhibitors, drug targets azoloazinesБиблиографические ссылки
Cheng H, Hoffman J, Le P, Nair SK, Cripps S, Matthews J, Smith C, Yang M, Kupchinsky S, Dress K, Edwards M, Cole B, Walters E, Loh C, Ermolieff J, Fanjul A, Bhat GB, Herrera J, Pauly T, Hosea N, Paderes G, Rejto P (2010) The development and SAR of pyrrolidine carboxamide 11β-HSD1 inhibitors. Bioorganic & Medicinal Chemistry Letters 20(9): 2897–2902. https://doi.org/10.1016/j.bmcl.2010.03.032 [PubMed]
Diedrich K, Krause B, Berg O, Rarey M (2023) PoseEdit: Enhanced ligand binding mode communication by interactive 2D diagrams. Journal of Computer-Aided Molecular Design 37(10): 491–503. https://doi.org/10.1007/s10822-023-00522-4 [PubMed] [PMC]
Du J, Qin W, Wen F, Zhao D, Yin X, Guo Z, Feng Q, Gu E, Pan Z, Wang L (2025) Luteolin’s potential in managing osteoporosis and bone metabolism disorders: Preclinical insights. Drug Design, Development and Therapy 19: 9715–9732. https://doi.org/10.2147/DDDT.S547141[PubMed] [PMC]
Flachsenberg F, Ehrt C, Gutermuth T, Rarey M (2024) Redocking the PDB. Journal of Chemical Information and Modeling 64(1): 219–237. https://doi.org/10.1021/acs.jcim.3c01573[PubMed]
Flachsenberg F, Meyder A, Sommer K, Penner P, Rarey (2020) A consistent scheme for gradient-based optimization of protein-ligand poses. Journal of Chemical Information and Modeling 60(12): 6502–6522. https://doi.org/10.1021/acs.jcim.0c01095 [PubMed]
Garcia J, Smith SS, Karki S, Drissi H, Hrdlicka HH, Youngstrom DW, Delany AM (2021) miR-433-3p suppresses bone formation and mRNAs critical for osteoblast function in mice. Journal of Bone and Mineral Research 36(9): 1808–1822.https://doi.org/10.1002/jbmr.4339 [PubMed]
Gillespie P, Pietranico-Cole S, Myers M, Bilotta JA, Conde-Knape K, Fotouhi N, Goodnow RA Jr, Guertin KR, Hamilton MM, Haynes NE, Liu B, Qi L, Ren Y, Scott NR, So SS, Spence C, Taub R, Thakkar K, Tilley JW, Zwingelstein C (2014) Discovery of camphor-derived pyrazolones as 11β-hydroxysteroid dehydrogenase type 1 inhibitors. Bioorganic & Medicinal Chemistry Letters 24(12): 2707–2711. https://doi.org/10.1016/j.bmcl.2014.04.049 [PubMed]
Graham FL, Smiley J, Russell WC, Nairn R (1977) Characteristics of a human cell line transformed by DNA from human adenovirus type 5. Journal of General Virology 36(1): 59–74. https://doi.org/10.1099/0022-1317-36-1-59 [PubMed]
Gu X, Dragovic J, Koo GC, Koprak SL, LeGrand C, Mundt SS, Shah K, Springer MS, Tan EY, Thieringer R, Hermanowski-Vosatka A, Zokian HJ, Balkovec JM, Waddell ST (2005) Discovery of 4-heteroarylbicyclo[2.2.2]octyltriazoles as potent and selective inhibitors of 11β-hsd1: novel therapeutic agents for the treatment of metabolic syndrome. Bioorganic & Medicinal Chemistry Letters 15(23): 5266–5269. https://doi.org/10.1016/j.bmcl.2005.08.052[PubMed]
Henderson B, Nair SP (2003) Hard labour: Bacterial infection of the skeleton. Trends Microbiology 11(12): 570–577. https://doi.org/10.1016/j.tim.2003.10.005[PubMed]
Henzler AM, Urbaczek S, Hilbig M, Rarey M (2014) An integrated approach to knowledge-driven structure-based virtual screening. Journal of Сomputer-Aided Molecular Design 28(9): 927–939. https://doi.org/10.1007/s10822-014-9769-4 [PubMed]
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. Journal of Molecular Graphics 14(1): 33–38. https://doi.org/10.1016/0263-7855(96)00018-5 [PubMed]
Koklin IS, Lebedev PR, Kochkarov AA, Gudyrev OS, Gureev VV, Dolzhikov AA, Taran EI, Korokin MV (2025) Regulation of 11β-hydroxysteroid dehydrogenase isoforms – novel drug targets for osteoporosis therapy. Research Results in Pharmacology 11(3): 109–114. https://doi.org/10.18413/rrpharmacology.11.807
LeBoff MS, Greenspan SL, Insogna KL, Lewiecki EM, Saag KG, Singer AJ, Siris ES (2022) The clinicianэs guide to prevention and treatment of osteoporosis. Osteoporosis International 33(10): 2049–2102. https://doi.org/10.1007/s00198-021-05900-y [PubMed] [PMC]
Ritz C, Baty F, Streibig JC, Gerhard D (2015) Dose-response analysis using R. PLOS ONE 10(12): e0146021. https://doi.org/10.1371/journal.pone.0146021 [PubMed] [PMC]
Robb GR, Boyd S, Davies CD, Dossetter AG, Goldberg FW, Kemmitt PD (2015) Design of pyrazolo-pyrimidines as 11β-HSD1 inhibitors through optimisation of molecular electrostatic potential. MedChemComm 6(5): 926–934. https://doi.org/10.1039/c5md00043b
Roche D, Carniato D, Leriche C, Lepifre F, Christmann-Franck S, Graedler U, Charon C, Bozec S, Doare L, Schmidlin F, Lecomte M, Valeur E (2009) Discovery and structure–activity relationships of pentanedioic acid diamides as potent inhibitors of 11β-hydroxysteroid dehydrogenase type I. Bioorganic & Medicinal Chemistry Letters 19(10): 2674–2678. https://doi.org/10.1016/j.bmcl.2009.03.140 [PubMed]
Ryu JH, Kim S, Han HY, Son HJ, Lee HJ, Shin YA, Kim JS, Park HG (2015) Synthesis and biological evaluation of picolinamides as potent inhibitors of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1). Bioorganic & Medicinal Chemistry Letters 25(3): 695–700. https://doi.org/10.1016/j.bmcl.2014.11.074 [PubMed]
Sander T, Freyss J, von Korff M, Rufener C (2015) DataWarrior: An open-source program for chemistry aware data visualization and analysis. Journal of Chemical Information and Modeling 55(2): 460–473. https://doi.org/10.1021/ci500588j [PubMed]
Schellhammer I, Rarey M (2007) TrixX: Structure-based molecule indexing for large-scale virtual screening in sublinear time. Journal of Computer-Aided Molecular Design 21(5): 223–238. https://doi.org/10.1007/s10822-007-9103-5 [PubMed]
Schöning-Stierand K, Diedrich K, Ehrt C, Flachsenberg F, Graef J, Sieg J, Penner P, Poppinga M, Ungethüm A, Rarey M (2015) ProteinsPlus: a comprehensive collection of web-based molecular modeling tools. Nucleic Acids Research 50(W1): W611–W615. https://doi.org/10.1093/nar/gkac305 [PubMed] [PMC]
Scott JS, deSchoolmeester J, Kilgour E, Mayers RM, Packer MJ, Hargreaves D, Gerhardt S, Ogg DJ, Rees A, Selmi N, Stocker A, Swales JG, Whittamore PR (2012) Novel acidic 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) inhibitor with reduced acyl glucuronide liability: the discovery of 4-[4-(2-adamantylcarbamoyl)-5- tert -butyl-pyrazol-1-yl]benzoic acid (AZD8329). Journal of Medicinal Chemistry 55(22): 10136–10147. https://doi.org/10.1021/jm301252n [PubMed]
von Korff M, Freyss J, Sander T (2008) Flexophore, a new versatile 3D pharmacophore descriptor that considers molecular flexibility. Journal of Chemical Information and Modeling 48(4): 797–810. https://doi.org/10.1021/ci700359j [PubMed]
Vuorinen A, Engeli RT, Leugger S, Kreutz CR, Schuster D, Odermatt A, Matuszczak B (2017) Phenylbenzenesulfonates and -sulfonamides as 17β-hydroxysteroid dehydrogenase type 2 inhibitors: synthesis and SAR-analysis. Bioorganic & Medicinal Chemistry Letters 27(13): 2982–2985. https://doi.org/10.1016/j.bmcl.2017.05.005 [PubMed]
Wahl J (2024) PheSA: An open-source tool for pharmacophore-enhanced shape alignment. Journal of Chemical Information and Modeling 64(15): 5944–5953. https://doi.org/10.1021/acs.jcim.4c00516 [PubMed]
Wang ZQ, Ovitt C, Grigoriadis AE, Möhle-Steinlein U, Rüther U, Wagner EF (1992) Bone and haematopoietic defects in mice lacking c-fos. Nature 360(6406): 741–745. https://doi.org/10.1038/360741a0 [PubMed]
Xia G, You X, Liu L, Liu H, Wang J, Shi Y, Li P, Xiong B, Liu X, Shen J (2013) Design, synthesis and SAR of piperidyl-oxadiazoles as 11β-hydroxysteroid dehydrogenase 1 inhibitors. European Journal of Medicinal Chemistry 62: 1–10. https://doi.org/10.1016/j.ejmech.2012.12.059 [PubMed]
Yang H, Dou W, Lou J, Leng Y, Shen J (2008) Discovery of novel inhibitors of 11β-hydroxysteroid dehydrogenase type 1 by docking and pharmacophore modeling. Bioorganic & Medicinal Chemistry Letters 18(4): 1340–1345. https://doi.org/10.1016/j.bmcl.2008.01.020 [PubMed]
Ye XY, Chen SY, Nayeem A, Golla R, Seethala R, Wang M, Harper T, Sleczka BG, Li YX, He B, Kirby M, Gordon DA, Robl JA (2011) Design, synthesis, and SAR studies of novel polycyclic acids as potent and selective inhibitors of human 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD-1). Bioorganic & Medicinal Chemistry Letters 21(22): 6699–6704. https://doi.org/10.1016/j.bmcl.2011.09.055 [PubMed]
Zhou M, An YZ, Guo Q, Zhou HY, Luo XH (2024) Energy homeostasis in the bone. Trends in Endocrinology and Metabolism 35(5): 439–451. https://doi.org/10.1016/j.tem.2023.12.009[PubMed]
Загрузки
Опубликован
Как цитировать
Выпуск
Раздел
Лицензия
Copyright (c) 2025 Korokin MV, Kotovskaya SK, Avtina TV, Koklin IS, Butorin II, Savateev KV, Urakov GV, Androv SV, Taran EI, Kochkarov AA, Korokina LV, Fedotov VV, Moseev TD, Gudyrev OS, Koroleva NV, Vasyutkin VV, Pykhtina PI

Это произведение доступно по лицензии Creative Commons «Attribution» («Атрибуция») 4.0 Всемирная.
 Русский
Русский
 English
English

