The excretion study of 4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonamide in rats
DOI:
https://doi.org/10.18413/rrpharmacology.11.524Abstract
Introduction: The 4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonamide (ODASA) is a newcarbonic anhydrase II inhibitor for open-angle glaucoma treatment, but the excretion study of this compound has not been performed yet. Aim: Calculation of excretion parameters of ODASAand its metabolites in urine and feces in rats.
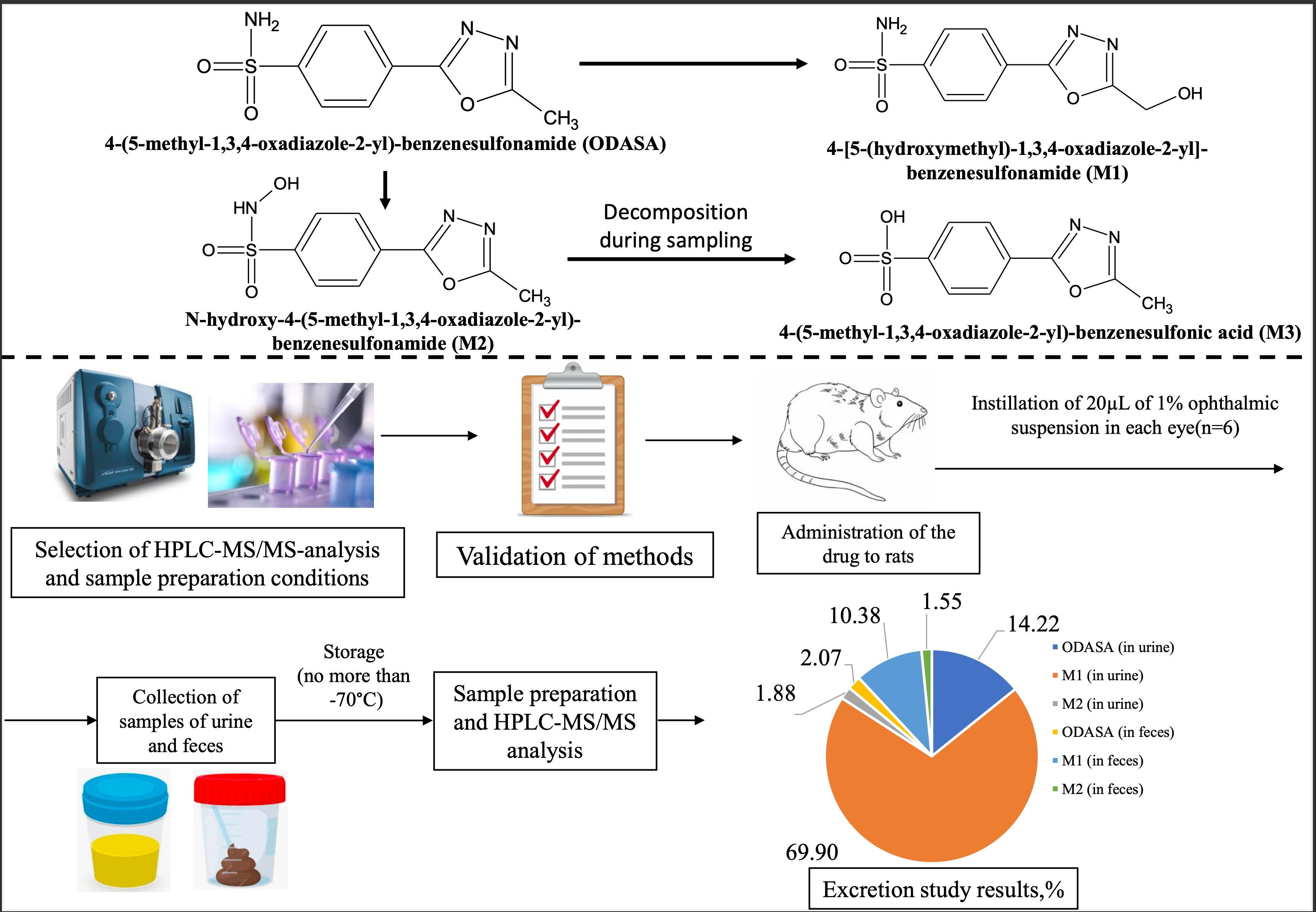
Materials and Methods: The ODASA excretion was investigated on 6 Wistar rats. The 1% suspension of ODASA was instilled into each eye in a volume of 20μL (1.6 mg/kg). Excreta were collected using metabolic cages. Sampling of feces was performed every 24 h for 384 h after the administration. Urine was taken frequently in first day of experiment: 4 h, 8 h and 12 h after administration. Samples was stabilized and frozen (temperature <-70°C). Quantification of ODASA, 4-[5-(hydroxymethyl)-1,3,4-oxadiazole-2-yl]-benzenesulfonamide (M1), N-hydroxy-4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonamide (M2), 4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonic acid (M3) was carried out by HPLC-ESI-MS/MS. M2 was unstable in samples, and its content was calculated by summing the concentrations of M2 and its degradation product M3. Kinetex Phenyl Hexyl column (50*4.6 mm, 2.6 µm) was used for chromatographic separation.
Results: The developed bioanalytical methods for rat excreta analysis were validated in the range of 10-10000 ppb for ODASA and M1, 1-1000 ppb – for M2, and 5-5000 ppb – for M3. Part of 16.29±1.60% of the active compound was eliminated in unchanged form, 80.27±1.68% – in the form of M1, and 3.43±0.33% – in form of M2 (М±SEM). The drug is mainly excreted by renal route: 14.22±1.43% in the form of ODASA, 69.90±1.80% – in the form of M1, and 1.88±0.24% – in the form of M2 (М±SEM). The highest rate of renal excretion of the studiedcompounds was observed in period of 8-12 hours after administration. The complete elimination of ODASA was achieved through 360 h after administration.
Conclusion: Most part of ODASA is eliminated in the form of M1. The main route of excretion is renal. The use of validated bioanalytical methods guaranteed reliability of the obtained data.
Graphical Abstract
Keywords:
HPLC-MS/MS, urine, feces, stabilization, validation, pharmacokinetics, excretion, rats, carbonic anhydrase II inhibitor, N-hydroxysulfonamideReferences
Almalki AH, Ali NA, Elroby FA, El Ghobashy MR, Emam AA, Naguib IA (2021) ESI–LC–MS/MS for therapeutic drug monitoring of binary mixture of pregabalin and tramadol: human plasma and urine applications. Separations 8(2): 21. https://doi.org/10.3390/separations8020021
Chen Y, Yang K, Liu S, Yu L, Wang R, Qin B (2025) Study on the excretion of a new antihypertensive drug 221s (2,9) in rats. Pharmaceuticals 18(8): 1138. https://doi.org/10.3390/ph18081138 [PubMed] [PMC]
Dong L, Liu W, Zhao X, Yu F, Xu Y, Su M (2022) Preclinical drug pharmacokinetic, tissue distribution and excretion profiles of the novel limonin derivate HY-071085 as an anti-inflammatory and analgesic candidate in rats and beagle dogs. Pharmaceuticals (Basel) 15(7): 801. https://doi.org/10.3390/ph15070801. [PubMed] [PMC]
Hu H, Xiao H, Bao H, Li M, Xue C, Li YT, Wang G, Chen S, Huang Y, Zheng L, Wang A, Li YJ, Gong ZP (2020) Tissue distribution comparison of six active ingredients from an eucommiae cortex extract between normal and spontaneously hypertensive rats. Evidence-Based Complementary and Alternative Medicine 2020: 2049059. https://doi.org/10.1155/2020/2049059 [PubMed] [PMC]
European Medicines Agency (2022) ICH Guideline M10 on Bioanalytical Method Validation And Study Sample Analysis, The Netherlands, Amsterdam, 45 pp.
Khokhlov AL, Yaichkov II, Shetnev A А, Korsakov MK, Volkhin N N, Petukhov SS, Tyushina A N, Lasaryanz O E (2024) The evaluation of pharmacokinetic parameters of 4-(5-methyl-1,3,4-oxadiazole-2-yl)-benzenesulfonamide and its metabolites in rat plasma. Research Results in Pharmacology 10(4): 67–76. https://doi.org/10.18413/rrpharmacology.10.523
G KK, Thaggikuppe Krishnamurthy P, Ammu V V V RK, Vishwanath K, Narenderan ST, Babu B, Krishnaveni N (2021) Development and validation of a sensitive LC-MS/MS method for pioglitazone: application towards pharmacokinetic and tissue distribution study in rats. RSC Advances. 11(19): 11437–11443. https://doi.org/10.1039/d1ra01126j [PubMed] [PMC]
Lu T, Wang X, Zhang Q, Liu K, Xu T, Wang Q, Zhao P, Cheng Z (2023) Validated LC-MS/MS method for quantitation of solasodine in rat urine and feces: Blocking nonspecific adsorption. Acta Chromatographica 35(4): 319–325. https://doi.org/10.1556/1326.2022.01079
Lu Y, Li N, Zhu X, Pan J, Wang Y, Lan Y, Li Y, Wang A, Sun J, Liu C (2021) Comparative analysis of excretion of six major compounds of Polygonum orientale L. extract in urine, feces and bile under physiological and myocardial ischemia conditions in rats using UPLC-MS/MS. Biomedical Chromatography 35 (10): e5174. https://doi.org/10.1002/bmc.5174 [PubMed]
Eurasian Economic Commission (2016) On Approval of the Rules for Conducting Bioequivalence Studies on Medicines in the Eurasian Economic Union. Decision of the Council of the Eurasian Economic Commission № 85 of November 3. [in Russian]
PangX, Zhao Y, Song J, Kang D, Wu S, Wang L, Liu A, Du G (2019) Pharmacokinetics, excretion and metabolites analysis of DL0410, a dual‑acting cholinesterase inhibitor and histamine-3 receptor antagonist. Molecular Medicine Reports 20(2): 1103–1112. https://doi.org/10.3892/mmr.2019.10306. Epub 2019 May 28. [PubMed] [PMC]
Qiu J, Zhu M, Wang Y, Chen B, Bai R, Chen F, Li Y, Zhou Y, Zhang L (2022) Pharmacokinetic and excretion study of eight active constituents in rat by LC-MS/MS after oral administration of the Toddalia asiatica extract. Analytical Biochemistry 640: 114407. https://doi.org/10.1016/j.ab.2021.114407 [PubMed]
Yaichkov II, Khokhlov AL, Korsakov MK, Volkhin NN, Petukhov SS, Zaykova VE, Lazariants OE (2025) Excretion study of 5-[5-(Trifluoromethyl)-1,2-oxazole-3-yl]-furan-2-sulfonamide in rats. Regulatory Research and Medicine Evaluation 15(5): 508–520. https://doi.org/10.30895/1991-2919-2025-697 [in Russian]
Yue J, Cheng W, Wei S, Liu G, ZhouM, Lv Z, Yu M (2023) Development and validation of UHPLC-MS/MS method for quantifying of agarotriose: An application for pharmacokinetic, tissue distribution, and excretion studies in rats. Journal of Ocean University of China 22: 1683–1691. https://doi.org/10.1007/s11802-023-5534-4
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Khokhlov AL, Yaichkov II, Shetnev AA, Korsakov MK, Volkhin NN

This work is licensed under a Creative Commons Attribution 4.0 International License.
 Русский
Русский
 English
English

